iMatrix-511 Recombinant Laminin E8 Fragments
- Cell culture matrix consisting of recombinant Laminin-511 E8 fragments.
- Sustains long term propagation of wide variety of cell types, especially pluripotent cells.
- Superior adhesion of human PSCs.
- Time-saving coating-free method.
- Superior for single cell cloning e.g. in CRISPR/Cas9 gene editing.
StemFit® Feeder-Free Stem Cell Culture Media
- Feeder-free stem cell culture media for iPS and ES cells.
- Weekend free culture, requiring fewer media changes.
- Animal-free and chemically defined.
- Optimised for single cell passaging with rapid expansion rates.
- Maintains high genetic stability over multiple passages.
- Available in research and GMP formulations.
CELLBANKER® Series Cryopreservation Media
- Long term storage of ESCs and iPSCs at -80˚C or -196˚C, and other fragile cell types.
- High viability - hiPSCs showed 98.1% cell viability upon thawing.
- No programmed freezing or liquid nitrogen required.
- Research and GMP-grade available.
- Custom formulations, containers, and volumes now available.
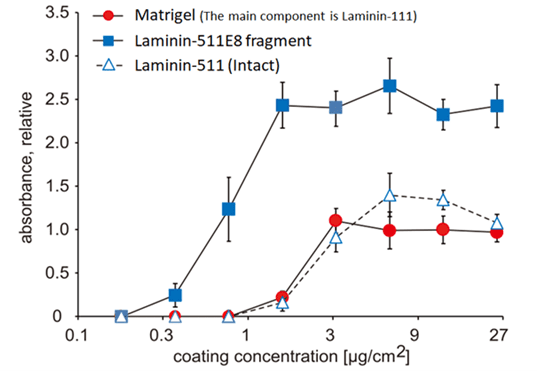
Superior cell culture with iMatrix-511 E8 fragments
Miyazaki et al (2012) showed that iMatrix Laminin-511E8 fragments demonstrate greater adhesion efficiency of dissociated cells than Matrigel or the intact Laminin-511 protein.
ABLE 3D Magnetic Stir and Disposable Bioreactor System for Stem Cell Culture
ABLE 3D Magnetic Stir and Disposable Bioreactor System is a 3D culture system designed specifically for the efficient and scalable cultivation of iPSC spheroid cultures. It combines magnetic stirring and disposable bioreactors to facilitate the expansion and differentiation of stem cells.
- Bioreactor capacity is 30 mL, enabling 5 to 10-fold cell expansion (up to 5 × 10⁷ cells)
- Typical growth is 200-300 μm spheroids
- Compatible with StemFit media and iMatrix-511 recombinant ECM for iPSC culture and expansion
NEW: STEM-CELLBANKER® EX
- Reliable and safe cryopreservation media for applications in advanced therapies, such as cell therapy.
- Animal component-free and chemically defined, with ingredients already approved for intravenous administration.
- No requirement for washing of the cryopreservation solution upon delivery of the cell therapy product.
- High cell viability post-thaw, and high retention of cell identity.

NEW: Small Molecules
Small molecules are cell permeable, organic compounds with low molecular weights, that can be used as tools to manipulate cell fates via the targeting of signalling pathways. This makes small molecules extremely useful in stem cell and regenerative medicine research. Our diverse range of small molecules, including A83-01, CHIR99021, DAPT, and valproic acid, possess the remarkable ability to promote self-renewal in stem cells, offering promising avenues for advancing your stem cell research.
Explore our range of small molecules, and advance your life science research today!
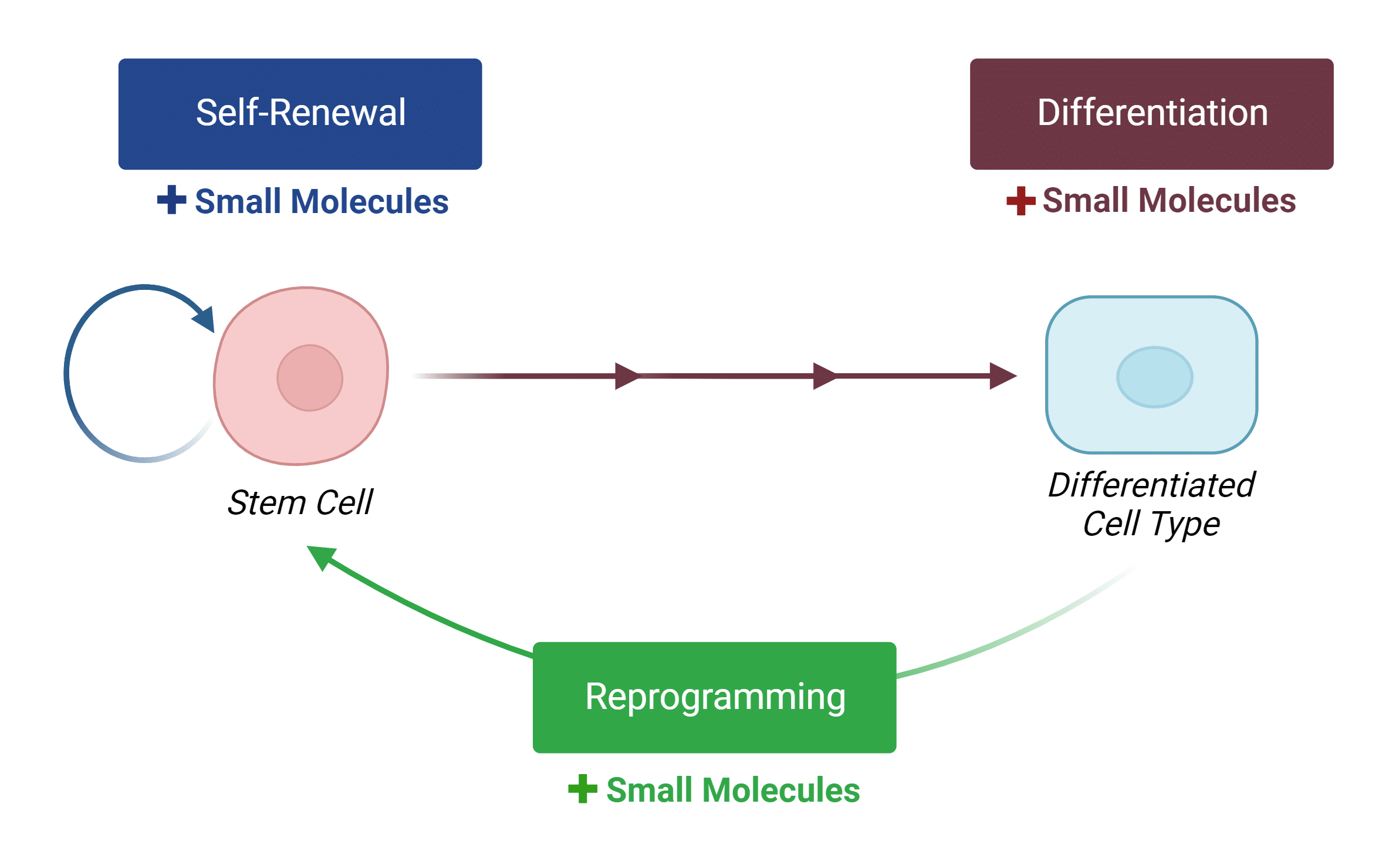
Stem Cell Synergy - Grow and Store products in world-leading research
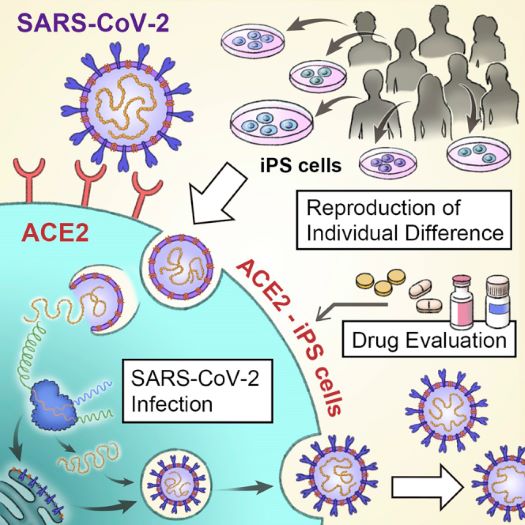
Modelling SARS-COV-2 infection using ACE2-expressing iPS cells
- Dr. Kazuo Takayama's group at Kyoto University have utilised Stem Cell Synergy products to reproduce the life cycle of the SARS-COV-2 virus in previously uninfectable human iPS cells by overexpressing the virus's binding site, ACE2.
- Differences in infection efficiency was observed between the ACE2-iPS cells donated from 8 different individuals, such as increased viral replication in male-derived cells. It is now possible to investigate other individual differences, such as genetic background, race, and blood type.
- The ACE2-iPSC model confirmed the efficacy of COVID-19 treatments such as Remdesivir, indicating that these ACE2-iPS cells could be used to evaluate future COVID-19 drug candidates.
“Although many cells are required for COVID-19 drug evaluation, iPS cells cultured with StemFit and iMatrix have a high replication capacity, so that sufficient cell numbers can easily be prepared – and can easily be cryopreserved as required using STEM-CELLBANKER."
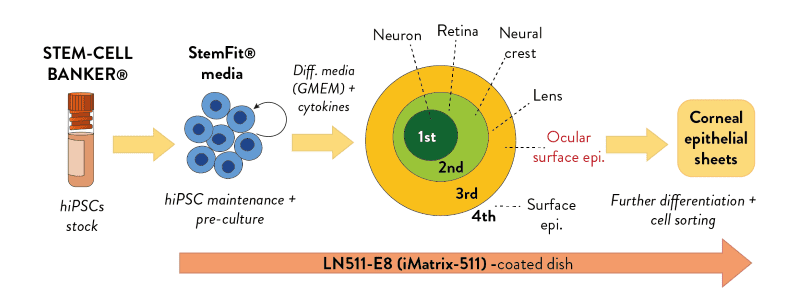
Generation of transplantable corneal epithelium from hiPSCs
- Professor Ryuhei Hayashi and colleagues at Osaka University in Japan have described a protocol using all Stem Cell Synergy products to generate transplantable corneal epithelium from human induced pluripotent stem cells (hiPSCs). This was achieved by isolating differentiated cells from specific concentric zones relating to different lineages of whole eye development, termed the SEAM.
- The researchers used StemFit medium for the pre-culture and maintenance of human iPSCs and the iMatrix-511 Laminin E8 fragments for both hiPSC culture and as a substrate for the growth of cells and tissues of the eye.
- Clinical trials began in 2019, where the first patient with corneal epithelial stem cell deficiency had a hiPSC-generated cornal epithelium transplant, to evalute safety and efficacy.
"Previously, feeder culture was performed, but the StemFit/Matrix-511 combination showed stable growth."
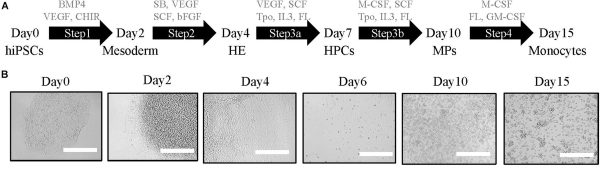
Serum-free differentiation of gene-edited human monocytes from hiPSCs
- Monocytes and macrophages play a vital role in our innate immune response, with impaired function of these cells being associated with both autoimmune and metabolic disorders. To advance treatments for these diseases, a reliable source of human monocytes is essential.
- At the Boehringer Ingelheim Pharmaceutical company, Dr Di Cui and colleagues have developed a serum-free protocol, using StemFit media and iMatrix-511 E8 fragments, to differentiate hiPSCs into human monocytes - with a 30-fold higher yield compared to the previous protocol.
- The study also highlights the feasibility of producing gene-edited hiPSC-derived macrophages by conducting CRISPR/Cas9 gene editing on hiPSCs and obtaining single KO clones, which can then undergo differentiation.
- Potential application: large scale production of therapeutic macrophages engineered wtih chimeric antigen receptors (CARs) to attack solid cancer tumours (Klichinsky et al. 2020).
Citations
A novel efficient feeder-free culture system for the derivation of human induced pluripotent stem cells.
Yamanaka, S. et al. Scientific Reports, 2014, 4, 359
An effective serum- and xeno-free chemically defined freezing procedure for human embryonic and induced pluripotent stem cells
Holm, F. et al. Human reproduction, 2010, 25(5), 1271-1279
Co-ordinated ocular development from human iPS cells and recovery of corneal function
Hayashi, R. et al. Nature, 2016, 531(7594), 376-380
Coordinated generation of multiple ocular-like cell lineages and fabrication of functional corneal epithelial cell sheets from human iPS cells.
Hayashi, R. et al. Nature Protocols, 2017, 12(4), 683-696
Efficient Adhesion Culture of Human Pluripotent Stem Cells Using Laminin Fragments in an Uncoated Manner.
Miyazaki, T. et al. Scientific reports, 2017, 7, 41165
High-Yield Human Induced Pluripotent Stem Cell-Derived Monocytes and Macrophages Are Functionally Comparable With Primary Cells
Cui, D. et al. Frontiers in cell and developmental biology, 2021, 9, 656867-656867
Modelling SARS-CoV-2 infection and its individual differences with ACE2-expressing human iPS cells.
Sano, E. et al. iScience, 2021, 24(5), 102428-102428