Features
- Ideal for feeder-free cell culture
- Provides superior adhesion of human ES and iPS cells
- Enables the passaging of single cells
- Compatible across a wide variety of cell types
- Makes it easy to achieve extended cultures of hES/hiPS cells
- Enables efficient bulk production
Why the E8 Fragment?
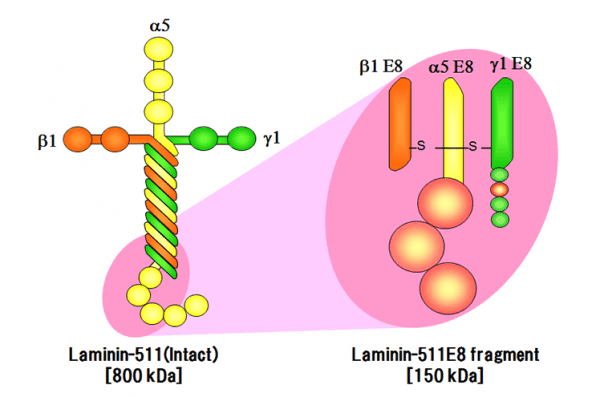
There are more than 12 Laminin isoforms, all of which promote stem cell growth. Laminin-511 is a major component of the basement membrane, which is expressed in early development of the embryo and can be used as a matrix for pluripotent (ES/iPS) stem cells, as it binds to integrin on cell surfaces. Laminin-511 is more efficient at enhancing cell growth than other Laminins such as Laminin-111 (Matrigel). However, Laminin-511 is a large protein (800kDa) composed of three chains (alpha, beta and gamma), making it difficult to produce recombinantly. In order to overcome this challenge, Laminin-511 proteins were fragmented to find the smallest integrin-binding component and hES cells were found to adhere more strongly to the E8 fragment than to the full-length protein.
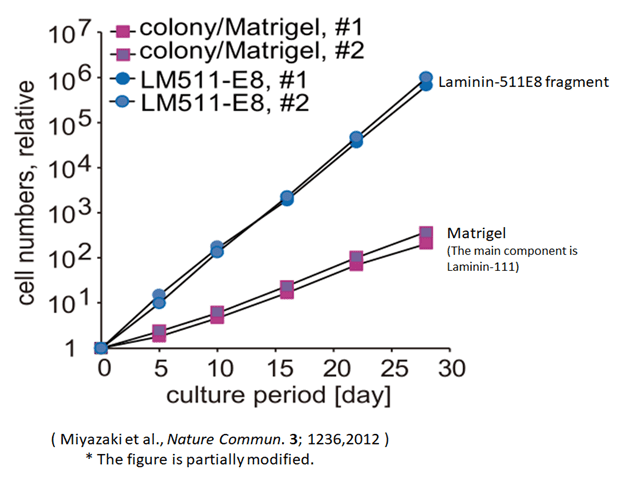
Culture More ES & iPS Cells
Figure 2 results show that the number of cells multiplied by 200 or more with the use of Laminin-511 E8 fragment in comparison with the conventional method (colony method).
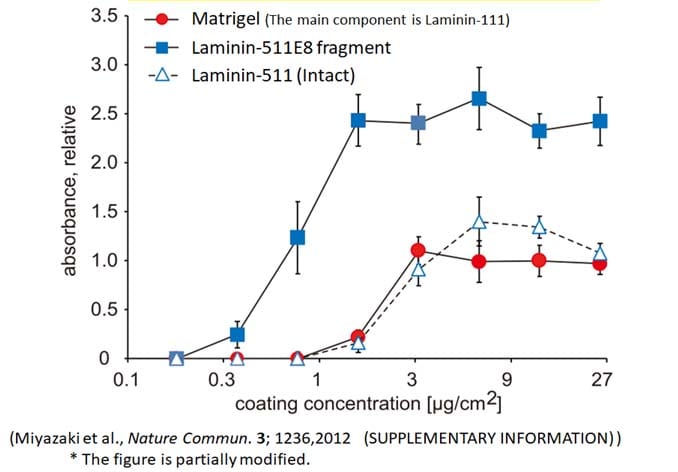
Superior Binding Activity
The Binding activity of Laminin 511 E8 Fragment against ES cell was better than Full length 511 and traditional substrate. The same function was confirmed in human iPS cells.

iMatrix-511
For maintenance and expansion of Pluripotent Stem Cells
A highly purified and refined product of human recombinant laminin-511 E8 fragment expressed by CHO-S cells. iMatrix-511 easily enables extended culture of human pluripotent stem cells and has swiftly established itself as the gold standard for stem cell culture.
The clinical grade version of iMatrix-511 is also available; iMatrix-511 MG meets the Standards of Biological Ingredients set by the PMDA, a Japanese government agency homologous to the FDA. To enquire about iMatrix™-511 MG, please email us at [email protected].

iMatrix-511 Silk
For maintenance and expansion of Pluripotent Stem Cells
iMatrix-511 Silk is human recombinant Laminin-511 E8 fragment with the same functions and performance as those of iMatrix-511, the only difference being is that it is expressed in transgenic silkworm cocoon rather than CHO-S cells. The highly productive transgenic silkworms make recombinant laminin fragment at a competitive price.
| Name | Datasheet | Packsize | Order |
|---|---|---|---|
| Recombinant Laminin iMatrix-511 silk | 1050ug (6 x 175ug tubes) | View |
Clinical Trial Data
Clinical trials using hPSC-derived cells have been initiated recently for various diseases, such as age-related macular degeneration (AMD), Parkinson’s disease (PD), spinal cord injury, and heart failure. Technology to produce quality cells is essential to the success of cell therapy.
Feeder cells and serum-derived components were previously used for the early stages of hPSC culture. However, current methods employ a combination of extracellular matrices (ECMs) and defined xeno-free (XF) and animal-origin-free (AOF) medium under feeder-free conditions to improve safety, consistency, and efficiency. Our AOF, weekend-free StemFit media has been used in several clinical trials alongside our iMatrix-511 as the cell culture substrate.
| ID Number | Year | Cell line | Derived cell type | Indication | Culture medium | Scaffold | Reference |
|---|---|---|---|---|---|---|---|
| UMIN000033564 | 2018 | iPSC (QHJI01s04, episomal) | Dopaminergic progenitors | Parkinson’s disease | AOF medium (StemFit) | Feeder-free (Laminin511E8) | Doi et al. (2020) |
| UMIN000036539 jRCTa050190084 | 2019 | iPSC (YZWJs524, episomal) | Corneal epithelial cell sheet | Limbal stem-cell deficiency | AOF medium (StemFit) | Feeder-free (Laminin511E8) | Hayashi et al. (2017) |
| UMIN000035074 jRCTa031190228 | 2020 | iPSC (YZWJs513, episomal) | Neural stem/progenitor cells | Spinal cord injury | AOF medium (StemFit) | Feeder-free (Laminin511E8) | Itakura et al. (2017) Sugai et al. (2016) |
| jRCTa050190104 | 2020 | iPSC (QHJI01s04, episomal) | Cartilage | Articular cartilage damage | AOF medium (StemFit) | Feeder-free (Laminin511E8) | Yamashita et al. (2020) |
| NCT04945018 | 2021 | iPSC (QHJI14s04, episomal) | Cardiomyocyte spheroids | Heart failure | AOF medium (StemFit) | Feeder-free (Laminin511E8) | Tohyama et al. (2017) |
| NCT04696328 UMIN00032989 | 2021 | iPSC (QHJI14s04, episomal) | Cardiomyocytes sheet | Ischemic cardiomyopathy | AOF medium (StemFit) | Feeder-free (Laminin511E8) | Miyagawa et al. (2022) Kawamura et al.(2016) |
Additional iMatrix™ products
iMatrix-411 (α4β1γ1) – induction of differentiation of vascular endothelial cells from ES/iPS cells
iMatrix-221 (α2β2γ1)– purification/maintenance culture of cardiomyocytes/skeletal muscle cells
iMatrix-332 (α3β3γ2)– induction of differentiation from iPS cells to corneal epithelial cells
iMatrix-111 (α1β1γ1)– induction of differentiation from human iPS cells to hepatoblast-like cells
iMatrix-511 Frequently Asked Questions
- Currently, it is sold only in liquid form. One tube contains 175 ug of cell culture substrate (laminin 511-E8 fragment) at a concentration of 0.5 mg / mL.
- The lyophilized version of iMatrixTM-511 has been discontinued by the manufacturer as of March 2015.
- The storage temperature of the product is refrigerated at 2-8 ° C
- Please refer to the table below for product expiration date.
| Expiration date | Applicable products |
|---|---|
| 2 years from date of production | iMatrixTM-511, iMatrixTM-511 silk, iMatrixTM-511MG, iMatrixTM-411, iMatrixTM-221 |
| 1 year and 6 months from date of production | Easy iMatrixTM-511, Easy iMatrixTM-511 silk |
| 6 months from date of production | Quick iMatrixTM-511, Quick iMatrixTM-511 silk |
[ The specific expiration date is printed on the product box. ]
- No. It can not be stored frozen.
- Purity is over 95%. Please refer to the lot specific data sheet for more information.
- Miyazaki et al. Nature communications, 3(1236), 1-10, 2012, and Scientific Reports, 7, 41165, 2017.
- The following are used.
・mTeSR1,TeSR2,TeSR-E8 (STEMCELL Technologies)
・StemProhESCSFM (Thermo Fisher Scientific)
・StemFitAK03 (Ajinomoto) - Nakagawa et al. Scientific Reports, 4(3594), 1-7, 2014.
The following are used.
・StemFit (Ajinomoto) - The publication stated that all media showed good results.
- The optimal coating concentration depends on the cell line and must be determined by the end user. We recommend first trying iMatrixTM at 0.5 ug / cm2 and consider the optimal concentration to be between 0.1 and 1.5 ug / cm2.
- In addition, there is also a new method for plating, the Pre-mix method:
Miyazaki et al. Scientific Reports, 7, 41165, 2017.
- Please refer to the link below for the expansion culture protocol of ES / iPS cells using iMatrixTM-511 and the movie of passage operation.
- Nakagawa et al. Scientific Reports, 4(3594), 2014.
- Use Rock Inhibitor at cell passage. However, it is not used during medium exchange.
- Yes. iPS cell passage can be handled with a single cell.
- Nakagawa et al. Scientific Reports, 4(3594), 2014.
- * Links to the external site*
- ((Expansion culture protocol)
- We recommend using trypsin.
- Nakagawa et al. Scientific Reports, 4(3594), 2014
- * Links to the external site.
((Expansion culture protocol))
- Currently, there is no data to support culture of mouse iPS cells using iMatrixTM-511.
- Matrigel contains laminin-111 from mouse EHS sarcoma. It also contains molecules other than laminin.
- iMatrixTM-511 is a recombinant protein purified from laminin 511-E8 fragment expressed in CHO-S cells with high purity.
- iMatrixTM-511 silk is a recombinant protein in which the laminin 511-E8 fragment is highly purified by silkworm-produced cocoons.
- Human ES cells and iPS cells are known to adhere to laminin-511 through cell membrane receptors (especially a6b1 integrin).
- Human ES cells and iPS cells are known to exhibit high adhesion activity to laminin-511.
This makes it possible to pass iPS cells into single cells and pass them with iMatrixTM-511 / iMatrixTM-511 silk. - In Miyazaki et al. (Nature communications, 3 (1236), 1-10, 2012), the expansion efficiency of the number of cells after 5 passages (after 30 days) is about 200 times higher than that of Matrigel.
Supporting Documents
Sanaki-Matsumiya, M., Matsuda, M., Gritti, N., Nakaki, F., Sharpe, J., Trivedi, V., & Ebisuya, M. (2022). Periodic formation of epithelial somites from human pluripotent stem cells. Nature Communications,13(1), 1-14.
Cites iMatrix-511 silk and StemFit® Basic04 CT culture medium; also Lipidure-CM5206 for low adhesion culture
Tanosaki, S., Akiyama, T., Kanaami, S., Fujita, J., Ko, M., Fukuda, K., & Tohyama, S. (2022). Purification of cardiomyocytes and neurons derived from human pluripotent stem cells by inhibition of de novo fatty acid synthesis. STAR Protocols, 3(2), 101360.
Cites iMatrix-511, iMatrix-221 and StemFit® Basic03 culture medium
Kihara, Y., Homma, J., Takagi, R., Ishigaki, K., Nagata, S., & Yamato, M. (2022). Laminin-221-derived recombinant fragment facilitates isolation of cultured skeletal myoblasts. Regenerative Therapy, 20, 147-156.
Cites iMatrix-221
Hwang, Y. S., Suzuki, S., Seita, Y., Ito, J., Sakata, Y., Aso, H., ... & Sasaki, K. (2020). Reconstitution of prospermatogonial specification in vitro from human induced pluripotent stem cells. Nature Communications, 11(1), 1-17.
Cites iMatrix-511 Silk with StemFit® Basic04 medium, with cell collection in CELLOTION and cryopreservation in CELLBANKER I
Aoki, H., Yamashita, M., Hashita, T., Iwao, T., & Matsunaga, T. (2020). Laminin 221 fragment is suitable for the differentiation of human induced pluripotent stem cells into brain microvascular endothelial-like cells with robust barrier integrity. Fluids And Barriers Of The CNS, 17(1).
Cites iMatrix-221, iMatrix-411 and iMatrix-511
Hayashi, R., Ishikawa, Y., Katori, R., Sasamoto, Y., Taniwaki, Y., & Takayanagi, H., ... & Nishida, K. (2017). Coordinated generation of multiple ocular-like cell lineages and fabrication of functional corneal epithelial cell sheets from human iPS cells. Nature Protcols, 12(4), 683-696.
Cites iMatrix-511 with StemFit® Basic03 medium, and cryopreservation in STEM-CELLBANKER
Miyazaki, T., Isobe, T., Nakatsuji, N., & Suemori, H. (2017).Efficient Adhesion Culture of Human Pluripotent Stem Cells Using Laminin Fragments in an Uncoated Manner. Scientific Reports, 7(1).
Cites iMatrix-511 and StemFit® Basic03 as cell culture medium
Ohta, R., Niwa, A., Taniguchi, Y., Suzuki, N., Toga, J., Yagi, E., ... & Saiko, M. (2016). Laminin-guided highly efficient endothelial commitment from human pluripotent stem cells . Scientific Reports, 6(1).
Cites iMatrix-511 and iMatrix-411