Our Services
Our CAR-T platform is highly adaptable to your needs and starting materials, as we can start with a target molecule (Phase I) or antibody (Phase II). We construct the single chain variable fragment (ScFv), transfer it into a CAR lentivector of your choice, make lentivirus and transduce activated human (or mouse) T cells. After the CAR-T cells proliferate, we measure the cytotoxicity in a real time assay, analyse CAR expression and quantitate cytokine production.
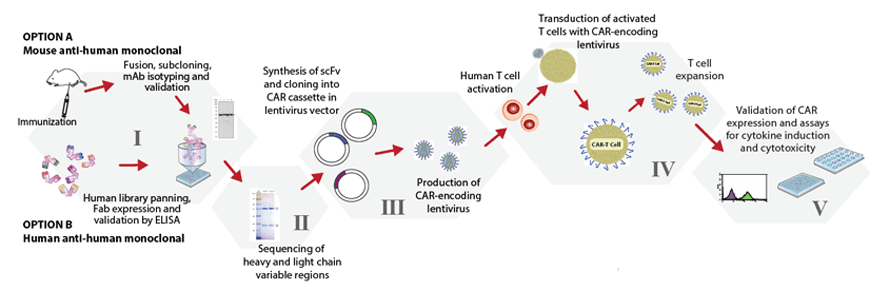
| Development Stage | Description | Estimated Time |
|---|---|---|
| Phase I |
| 25 weeks |
| Phase II |
| 4 weeks |
| Phase III |
| 4 weeks |
| Phase IV |
| 3 weeks |
| Phase V |
| 3 weeks |
Custom CAR-T Request Form

Let's get started
Complete our service request form and we will contact you to discuss your requirements.