CELLBANKER® Cell Freezing Media
Cryopreservation solution for cells and tissues
The CELLBANKER® series of cell freezing media allows for the stable long-term storage of cells. With its unique formulation which enables stable cryopreservation and high viability after freeze-thaw procedures, CELLBANKER is a trusted solution for the storage of any cell type including sensitive cell lines.
Available in several formulations, this series of easy-to-use cell freezing media offers high cell viability in serum, serum-free, GMP and DMSO-free formats.
Benefits
Cited in over 500 scientific publications, CELLBANKER® is a trusted solution for the storage of any cell types.
- Enables long term cell storage for >8 years at -80˚C or -196˚C
- Consistent high cell viability (>90%)
- Serum, serum-free and chemically defined formulations
- Ready-to-use formulation with a simple protocol
- Tested on many cells
- No programmed freezer or liquid nitrogen required
- Long shelf life (3 years from manufacturing date)
CELLBANKER® 1
Serum containing freezing medium
The first product of the CELLBANKER® series, CELLBANKER® 1, was launched in 1992 and now has a significant history of reliable, consistent and high viability recoveries post-cryopreservation. Contains serum, DMSO, glucose, salts and buffer.

CELLBANKER® 2
Serum free freezing medium
A serum free freezing medium that allows cell cryopreservation directly at -80°C without requiring a rate controlled freezer. Contains no animal-derived products and is guaranteed sterile.
Custom CELLBANKER®
Tailored cryopreservation storage solutions for cell therapy manufacturing
Introducing Custom CELLBANKER® from AMSBIO – the ideal GMP-grade cryopreservation solution for scaling up from research to manufacturing. Tailor the formulation, volume, and container type to your needs to ensure seamless integration into large-scale cell therapy product manufacturing and storage to match your workflow and regulatory requirements. Contact us to discuss your cryopreservation requirements and streamline your strategy with Custom CELLBANKER®.
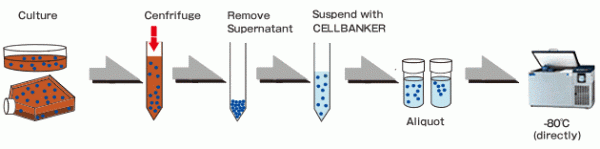
Cryopreservation Procedure
CELLBANKER® solutions are simple to use. Achieve the highest cell viability whilst maintaining stem cell pluripotency, normal karyotype and proliferation ability after cryopreservation using CELLBANKER®.
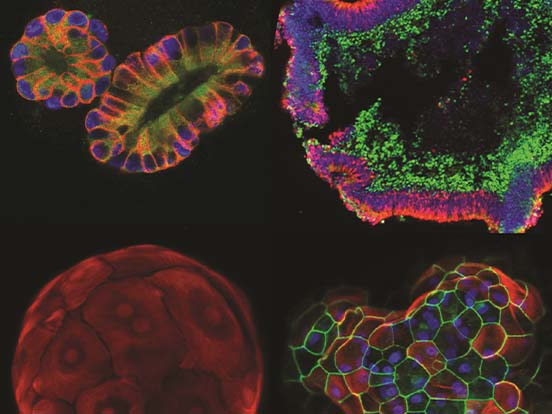
Cryopreservation of Organoids
Organoids are 3D, organ-like structures derived in vitro via self-assembly of pluripotent stem cells or adult tissue stem cells. CELLBANKER® media has been validated for cryopreservation and recovery of whole organoids, with high viability rates. Clones with desired genetic characteristics (e.g. CRISPR/Cas9 edits) frozen in CELLBANKER® media have also been successfully thawed and grown into organoids.
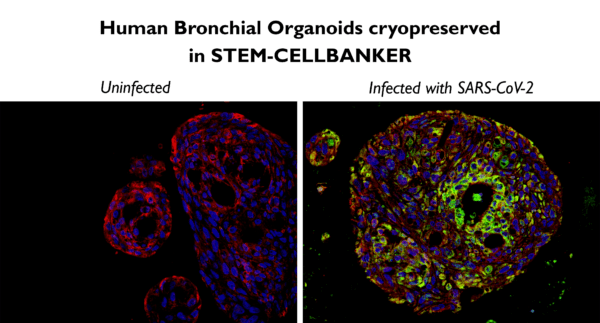
STEM-CELLBANKER® is GMP compliant, for applications of organoids in regenerative medicine, disease modelling and drug discovery. For example, Dr. Kazuo Takayama at the Center for iPS Cell Research and Application (CiRA) in Japan used STEM-CELLBANKER® to cryopreserve human bronchial organoids, as shown in Fig. 1 below.
Click here for customer testimony from the Clevers Lab, and click here to find out more about our organoid culture and storage solutions.
CELLBANKER 1:
Clevers lab (Hubrecht Institute, Netherlands) – Long term cryopreservation using CELLBANKER 1 of different types of organoids derived from cells of the airway and the thyroid to study associated diseases.
Lamers, M. M. et al. (2021), The EMBO journal, 40(5), e105912.
van der Vaart, J. et al. (2021), EMBO reports, 22(12), e52058.
(J. van der Vaart, personal communication)
Mayhew Lab (Cincinnati Children’s Hospital, USA) – Cryopreservation of pellets of hPSC-derived mid-hindgut endoderm (MHE), subsequently aggregated into small intestinal organoids (HIOs).
Pitstick, A. L. et al. (2022). Stem Cell Reports, 17(8), 1889-1902.
CELLBANKER 2:
Vallier lab (Cambridge, UK) - Cryopreservation of cholangiocyte organoids.
Tysoe, O. C. et al. (2019). Nat Protoc, 14(6), 1884-1925.
Stuart Forbes (Centre for Regenerative Medicine, Institute for Regeneration and Repair, University of Edinburgh, UK) - Freezing primary liver cells from tissue samples and also the organoids they culture from these cells.
Hallett, J. M. et al. (2022), Cell stem cell, 29(3), 355-371.e310
Knoblich lab (Vienna, Austria) - Archiving clones with desired genetic characteristics.
Bagley, J. A. et al. (2017), Nature Methods, 14(7), 743-751.
Bajaj, S. et al. (2021), The EMBO Journal, 40(23), e108714.
Joost Gribnau and Bas van Rijn (Erasmus University Medical Center, Netherlands) – Cryopreservation of Trophoblast Organoids.
Schäffers, OJM, et al. Organoids 1.2 (2022): 106-115.
STEM-CELLBANKER:
Takayama lab (CiRA, Japan) - Cryopreservation of SARS-CoV-2 model organoids.
Sano, E. et al. (2022), Communications Biology, 5(1), 516.
Allon Klein lab (Harvard, USA) - Cryopreservation of Intestinal organoids.
Tallapragada, N. P. et al. (2021), Cell Stem Cell, 28(9), 1516-1532.e1514.
Takenaka lab (Osaka International Cancer Institute, Japan) - Cryopreservation of Organoids from Human Epithelioid Sarcoma.
Wakamatsu, T. et al. (2022), Front Oncol, 12, 893592.
Frequently Asked Questions
Which types of cell lines can be cryopreserved with CELLBANKER®?
Almost all the cell lines can be cryopreserved by CELLBANKER®. View our list of cell types tested with CELLBANKER® and STEM-CELLBANKER® for more details.
What is the difference between CELLBANKER®1, and 2?
CELLBANKER® 1 contains serum; whereas CELLBANKER® 2 is a serum and protein- free type of medium.
What kind of serum is used? Which country does the serum origin?
New Born Calf Serum (NBS) is principally used and its origin is in Australia or New Zealand.
Why is programming freezing not needed for CELLBANKER® cryopreservation?
CELLBANKER® is formulated only for deep- freezer rapid freezing use at approximately -85℃. Freezing procedures by programming freezer or liquid nitrogen may deteriorate the cell viability. Storage of cells in a liquid nitrogen tank is recommended only after a sufficient freeze by deep freezer.
Tell me more about the components? Is dimethylsulfoxide (DMSO) included?
Unfortunately, the components of CELLBANKER® are not available to release at this time. DMSO is used as a frozen protectant in the product. However, DMSO rarely has any effects on the cryopreservation. May we remind you to wash your cells sufficiently after thawing.
Can I preserve my normal cells , lymphocytes or stem cells with CELLBANKER®?
There are some types of cell that are not suitable to be preserved with CELLBANKER®. Therefore, an initial test prior to the actual freezing is recommended.
How can I store CELLBANKER®?
Generally, CELLBANKER® is recommended to be kept at 4℃ and should be completely used as soon as possible. If storing for 3 months or longer, separate into aliquot and keep it frozen (-20℃). To prevent from deterioration of the product, frequent freeze-thaw method (more than 3 times) should be avoided.
Is CELLBANKER® a medicinal product?
CELLBANKER® is for research use only. Therefore, it is prohibited to be use in medical practice.
Featured Citations
Human biliary epithelial cells from discarded donor livers rescue bile duct structure and function in a mouse model of biliary disease.
Hallett, M. et al. (2022), Cell stem cell, 29(3): 355-371
Immature mDA Neurons Ameliorate Motor Deficits in a 6-OHDA Parkinson's Disease Mouse Model and are Functional after Cryopreservation.
Leitner D et al. (2019) Stem Cell Research, 101617
Derivation of enteric neuron lineages from human pluripotent stem cells.
Barber K et al. (2019) Nature Protocols. Volume 14, 1261–1279
PD-1 signaling modulates interferon-γ production by Gamma Delta (γδ) T-Cells in response to leukemia.
Hoeres T et al. (2019) OncoImmunology. 1869:pp 1–16
Establishment and Culture of Patient-Derived Primary Medulloblastoma Cell Lines.
Badodi S et al. (2019) Methods Mol Biol. Volume 8, 2019 - Issue 3
Effect of cryopreservation on the appearance and liver function of hepatocyte-like cells in cultures of cirrhotic liver of biliary atresia.
Yamazaki T et al. (2018) In Vitro Cell Dev Biol Anim. Volume 54, Issue 6, pp 401–405
Exosomes derived from clinical-grade oral mucosal epithelial cell sheets promote wound healing.
Sjöqvist Set al. (2018) Journal of Extracellular Vesicles Volume 8, 2019 - Issue 1
Long-term Cryopreservation of Human and other Mammalian Cells at- 80° C for 8 Years.
Miyamoto. Yet al. (2018) Cell Medicine Volume 10: 1-7
mRNA vaccine-elicited antibodies to SARS-CoV-2 and circulating variants
Wang Z et al. (2021) Nature. 592, pages616–622
Advanced cirrhosis drives enhanced B-cell differentiation resulting in hyperglobulinemia.
Hiroyoshi. D. et al. (2018) Hepatology
Generation of Fabry cardiomyopathy model for drug screening using induced pluripotent stem cell-derived cardiomyocytes from a female Fabry patient.
Kuramoto. Y. et al. (2018) Journal of Molecular and Cellular Cardiology Volume 121, Pages 256–26
Fused cerebral organoids model interactions between brain regions.
Bagley. J. A. et al. (2017) Nature Methods. doi:10.1038/nmeth.4304
A novel efficient feeder-free culture system for the derivation of human induced pluripotent stem cells.
Yamanaka S et al. (2014) Scientific Reports 4 Article, number:359
Monolayer culturing and cloning of human pluripotent stem cells on laminin-521–based matrices under xeno-free and chemically defined conditions.
Rodin et al. (2014) Nat Protoc. 2014 Oct;9(10):2354-68
Transplantation of cultured dental pulp stem cells into the skeletal muscles ameliorated diabetic polyneuropathy: therapeutic plausibility of freshly isolated and cryopreserved dental pulp stem cells
Hata et al. (2014) Nat Protoc. 2014 Oct;9(10):2354-68
Cryopreservation of Induced Pluripotent Stem Cells
Miyamoto Y et al. (2012) Cell Medicine. Vol. 3, pp. 89–95, 2012
An effective serum- and xeno-free chemically defined freezing procedure for human embryonic and induced pluripotent stem cells.
Holm et al. (2010) Hum Reprod. 25(5):1271-9. Epub 2010 Mar 5.
Biological impact of xeno-free chemically defined cryopreservation medium on amniotic epithelial cells.
Miki T et al. (2016) Stem Cell Research & Therapy 2016 Jan 12;7:8
Improved cryopreservation of human hepatocytes using a new xeno free cryoprotectant solution
Saliem M et al. (2012) World J Hepatol. May 27;4(5):176-83.
Human primary cultured hepatic stellate cells can be cryopreserved
Nakamura A et al. (2010) Med Mol Morphol. 43:107–115
CELLBANKER 1:
Clevers lab (Hubrecht Institute, Netherlands) – Long term cryopreservation using CELLBANKER 1 of different types of organoids derived from cells of the airway and the thyroid to study associated diseases.
Lamers, M. M. et al. (2021), The EMBO journal, 40(5), e105912.
van der Vaart, J. et al. (2021), EMBO reports, 22(12), e52058.
(J. van der Vaart, personal communication)
Mayhew Lab (Cincinnati Children’s Hospital, USA) – Cryopreservation of pellets of hPSC-derived mid-hindgut endoderm (MHE), subsequently aggregated into small intestinal organoids (HIOs).
Pitstick, A. L. et al. (2022). Stem Cell Reports, 17(8), 1889-1902.
CELLBANKER 2:
Vallier lab (Cambridge, UK) - Cryopreservation of cholangiocyte organoids.
Tysoe, O. C. et al. (2019). Nat Protoc, 14(6), 1884-1925.
Stuart Forbes (Centre for Regenerative Medicine, Institute for Regeneration and Repair, University of Edinburgh, UK) - Freezing primary liver cells from tissue samples and also the organoids they culture from these cells.
Hallett, J. M. et al. (2022), Cell stem cell, 29(3), 355-371.e310
Knoblich lab (Vienna, Austria) - Archiving clones with desired genetic characteristics.
Bagley, J. A. et al. (2017), Nature Methods, 14(7), 743-751.
Bajaj, S. et al. (2021), The EMBO Journal, 40(23), e108714.
STEM-CELLBANKER:
Takayama lab (CiRA, Japan) - Cryopreservation of SARS-CoV-2 model organoids.
Sano, E. et al. (2022), Communications Biology, 5(1), 516.
Allon Klein lab (Harvard, USA) - Cryopreservation of Intestinal organoids.
Tallapragada, N. P. et al. (2021), Cell Stem Cell, 28(9), 1516-1532.e1514.
Takenaka lab (Osaka International Cancer Institute, Japan) - Cryopreservation of Organoids from Human Epithelioid Sarcoma.
Wakamatsu, T. et al. (2022), Front Oncol, 12, 893592.