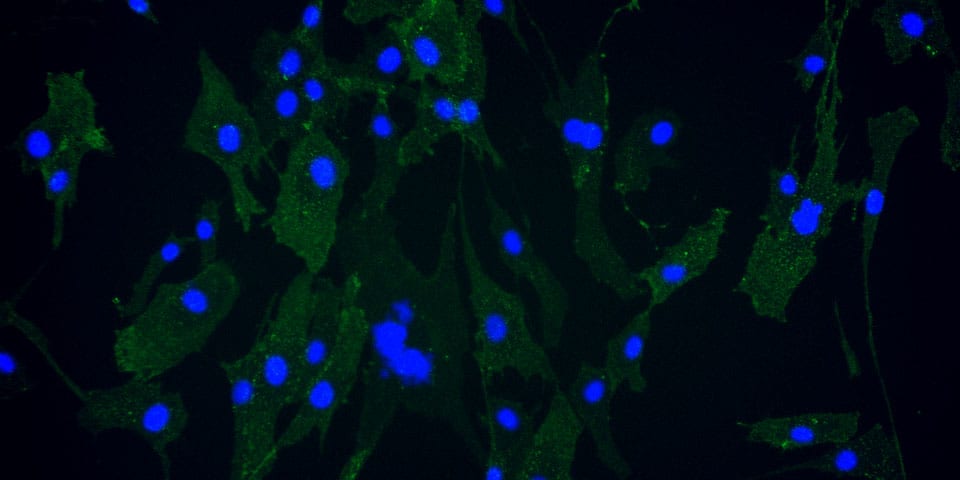
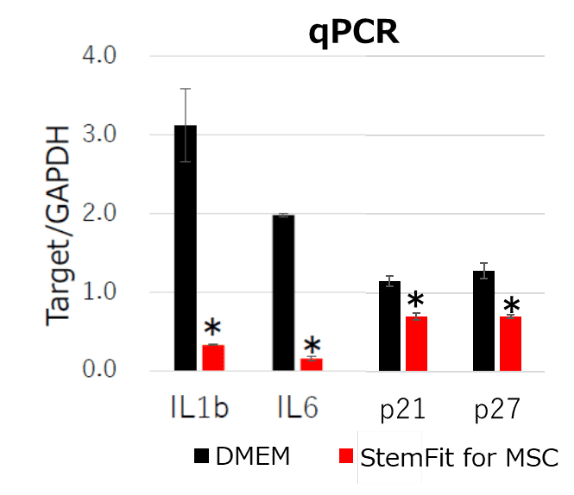
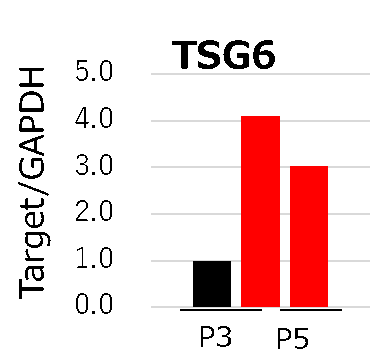
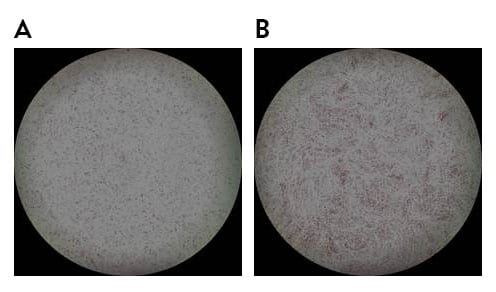
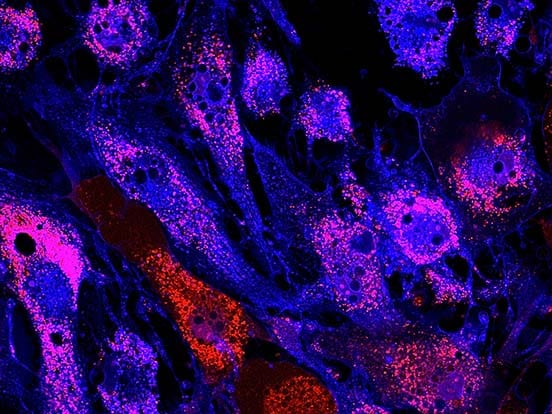
Dr. Takami and his colleagues use recombinant iMatrix-511 as extracellular matrix with StemFit® For MSC as culture medium to maintain Mesenchymal Stem Cells (MSCs), as recommended in our product information and protocols.
In the world’s first liver regeneration therapy using autologous bone marrow cells in November 20031, about 400 mL of bone marrow fluid collected under general anesthesia was used to infuse an uncultured whole bone marrow mononuclear cell fraction. After this, through joint researches in Japan and overseas, the safety and efficacy of the treatment were proved, and this treatment is now approved as advanced medical treatment. However, it is highly invasive to collect 400 mL under general anesthesia, so since 2008, they have been engaged in research on less invasive liver regeneration therapy in which MSCs are cultured and infused from a small amount of autologous bone marrow fluid.
Initially, the cultured cells were infused through a peripheral vein and the safety was confirmed, but the effective period was not very long. Therefore, the treatment method was improved, and a bridging study confirmed that it was better to improve the liver function by directly infusing the cells from the hepatic artery into the liver.