Skeletal Muscle Differentiation Kit
Differentiate human pluripotent stem cells to skeletal muscle
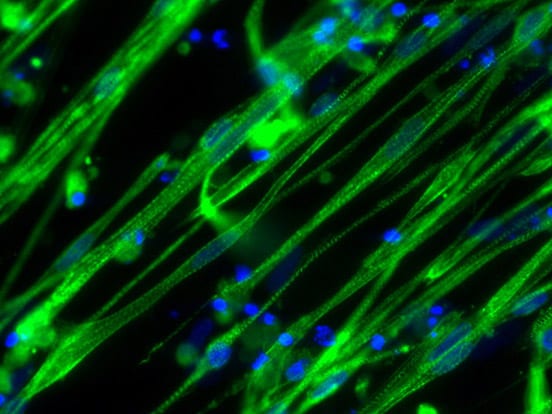
The world’s first protocol to differentiate human pluripotent stem cells to skeletal muscle with high yields and without cell sorting or genetic manipulation. Myotubes (muscle cells) are contractile, express typical muscle markers and show striated sarcomeres. The process yields almost pure populations of myogenic cells; it is highly efficient and works robustly with all pluripotent stem cell lines – critical prerequisites for effective cell-based disease modeling and drug development.
Benefits
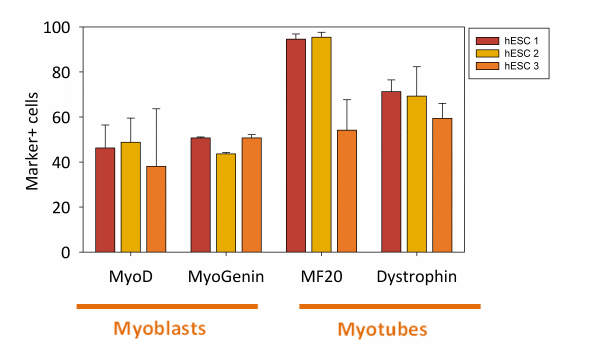
- Achieves typically 70% MF20-positive myotubes
- Simple 3-step process of media change and cell passaging
- No cell sorting steps required
- No transfection of myogenic transcription factors
- Tested on a wide range of embryonic & induced pluripotent stem cell lines
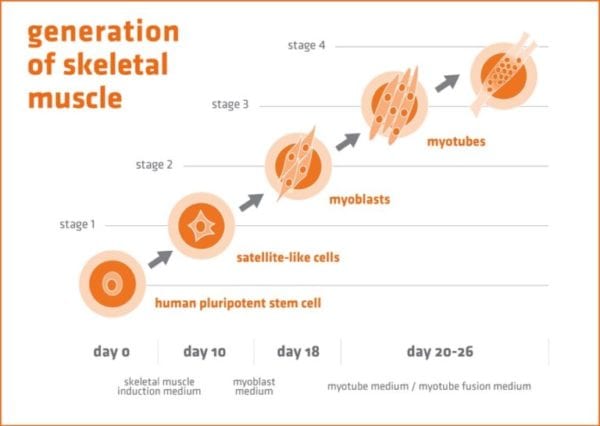
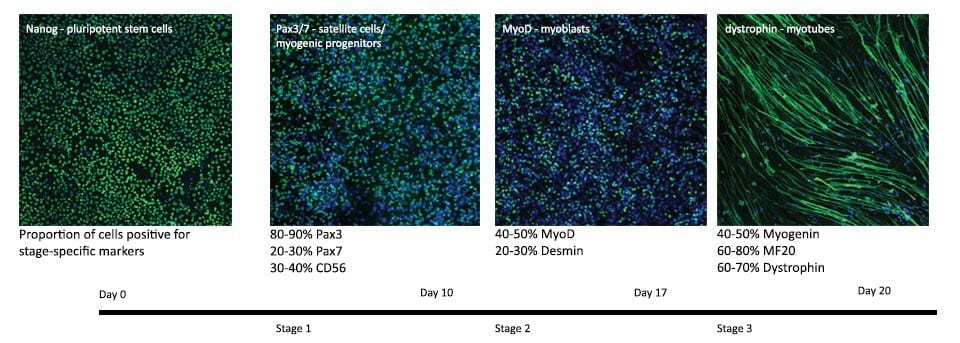
Generation of Muscle Cells in 3 Steps
Muscle is generated from human pluripotent stem cells in 3 steps, via satellite-like progenitor cells and myoblasts that then fuse to multinucleated myotubes in the third step. This third generation of the process includes an optional enhanced fusion formulation, depending on your desired phenotype.
Skeletal Muscle Differentiation Kit FAQs
To date we do not have any data to support the differentiation of non-human mammalian cells, however we have no information to suggest this application would not work.
To date we have not tested muscle tissue isolate myosatellite cells, however we do not have any evidence to suggest it is not possible to successfully differentiate myosatellite cells. However, myosatellite cells are highly proliferative and relatively rare, therefore a good dissociation protocol and sufficient tissue quantity would be required to yield enough cells to input into the differentiation protocol.
Our customers have reported successful differentiation on alternative commercial ECM, including iMatrix Laminin 511 E8 (Cat. #AMS.892 011) and Matrigel.
Yes, precursors and myoblasts can be banked and thawed as needed. These cells are proliferative but we recommend generating large banks starting with pluripotent cells through stage I and/or II. The cells can be dissociated with trypsin/EDTA and preserved in either stage medium with 10% DMSO or CELLBANKER® 1 (Cat. #11888).
The expression of satellite cell markers Pax3 and Pax7 peaks around day 4/5 of stage 1 differentiation. In addition to those markers we have verified expression of CD56, CD34 and CXCR4. See Caron et al. 2016.
Mature myotubes produced by the skeletal muscle differentiation kit can be maintained in the Myotube Cell Culture Medium for 2 weeks and the Myotube Fusion Medium for 3 weeks. However, mature myotubes will not passage well and at a certain point of maturation the cells will start to spontaneously contract which will cause them to detach from the ECM. Mature myotubes are not highly proliferative and cannot be easily frozen and recovered from cryopreservation. We recommend users cryopreserve their cells at either the progenitor or myoblast stage.
We are in the early stages of development on a culture system that will support long term culture of mature myotubes. It is based on a co-differentiation system in which the myotubes are innervated.
The AntiMHC by DSHB is a good marker for mature myotubes. Dystrophin is also a good marker.
The myotube fusion media produces more robust myotubes with a higher degree of cell fusion. Myoblasts continue to proliferate in Myotube Cell Culture Medium and the fusion rate is slower.
To date, we do not have any data to support the differentiation of MSCs to myotubes.
To date, we do not have any data to support the trans-differentiation of fibroblasts to myotubes.
The media is stable for 2 weeks at +4°C. It is also validated for one additional freeze/thaw cycle so it can be aliquoted & frozen and used by the printed expiration.