UltraMAB®, the Ultra Specific Antibody
Validated against >10,000 human antigens
Performance and specificity are the pre-requisites for antibodies to be used for diagnostic and therapeutic applications. AMSBIO supplies UltraMAB®, a unique line of ultraspecific and extensively validated monoclonal antibodies, to ensure superior performance in these areas.
Some commonly used diagnostic antibodies perform well in applications but cross-react with other unrelated proteins. This cross-reactivity may potentially cause unexpected side effects and generate false diagnostic reports for clinicians. However, the tools to test the mono-specificity are lacking and remain a challenge to the antibody market. A high density protein microarray chip has been developed for antibody specificity testing, and has been applied to identify UltraMAB® antibodies.
Features of UltraMAB® Antibodies
- Ultra-specific antibodies for cancer biomarkers and other important diagnostic targets
- Validated using High-Density Protein Microarray
- Validated against more than 10,000 human antigens
- Application validated for:
- WB with cell lines and tissue lysates
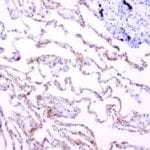
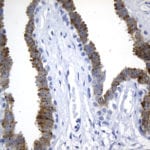
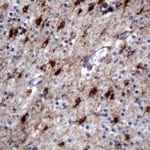
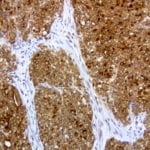
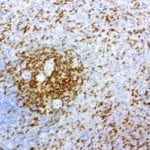
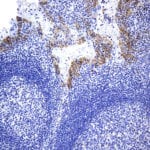
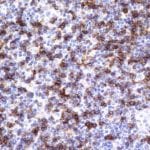
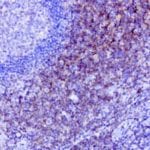
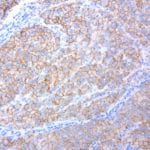
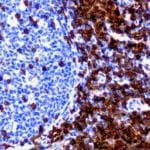
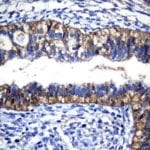
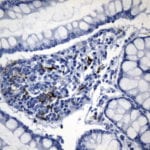
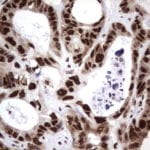
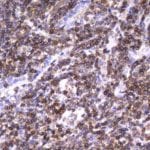
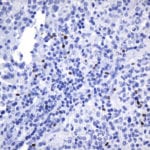
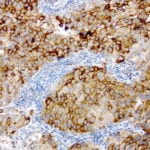
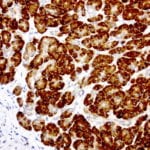
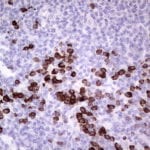
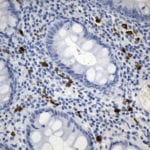
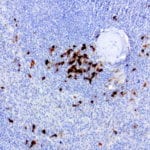
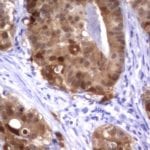
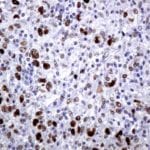
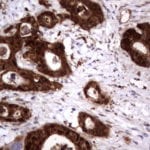
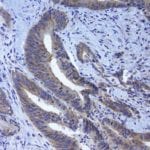
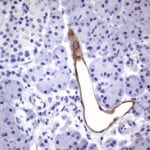
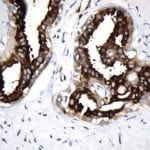
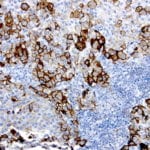
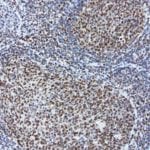
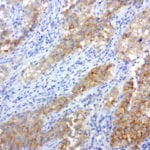
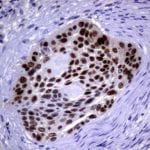
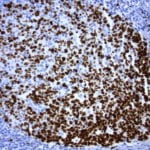
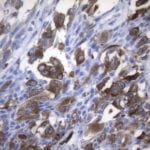
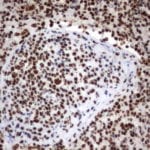
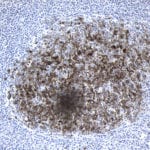
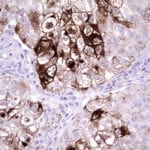
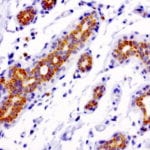
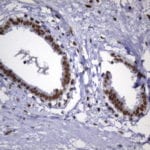
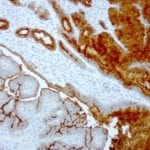
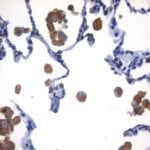
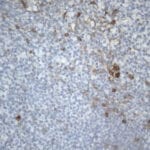
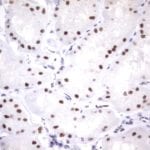
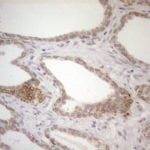
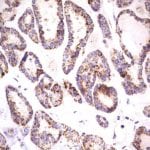
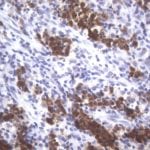
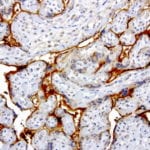
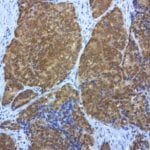
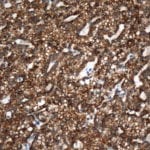
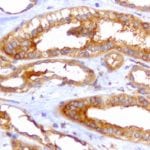
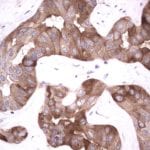
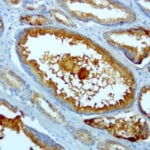
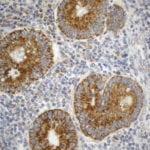
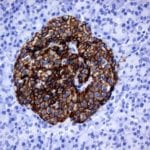
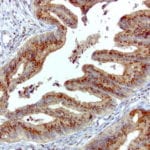
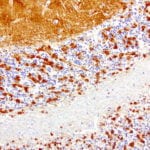
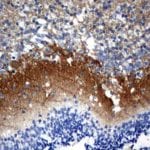
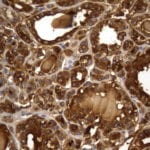
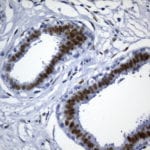
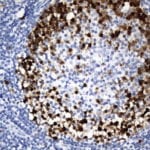
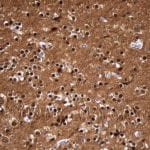
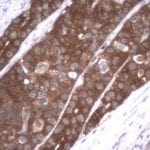
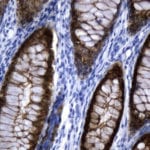
- IHC staining with over 25 types of normal and cancer human tissues
- IF/ICC
- FACS
- Mono-specificity test
High-Density Protein Microarray Platform
Under current specifications, 11,088 samples were spotted in duplicates on a single nitrocellulose slide. These include 10,380 unique overexpression lysates and 708 positive and negative control samples. Serial diluted mouse, human and rabbit IgG mixture as positive controls were spotted on three subarrays highlighted in red boxes. Subarrays highlighted in yellow boxes contain serial diluted HEK293T cell lysates as negative controls. All the subarrays contain standard positive and negative controls as shown in the figure.
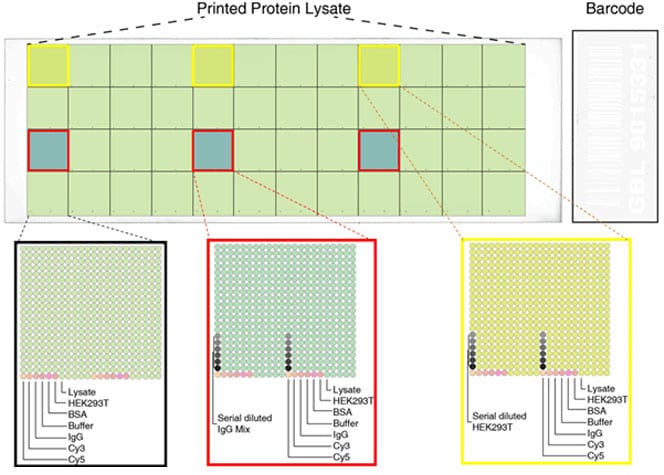
Protein Microarray Slide Specifications
| Slide Type: | White nitrocellulose glass slide |
| Slide NC pad dimension: | 20mm x 60mm |
| Total subarrays: | 48 (4 columns x 12 rows) |
| Subarray Size: | 4200um x 4400um |
| Subarray Dimensions: | 21 rows (Vertical) x 22 columns (Horizontal) |
| Median Spot Diameter: | ~120um |
| Spot Center to Center Spacing: | 200um |
| Distances Between Subarrays: | 100um |
| Replicates per Sample: | 2 |
The high density microarray platform, has been used to validate the specificity of existing HER2 and ERCC1 diagnostic antibodies as well as identify UltraMAB® antibodies.
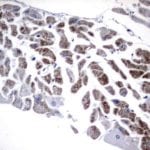
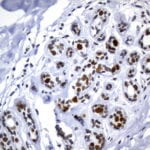
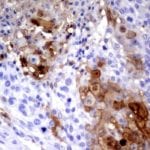
Case Study 1: HER2 Pathology Antibodies
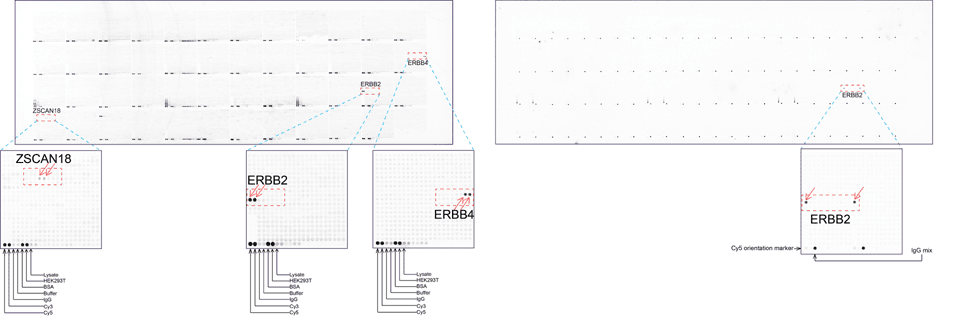
"A semi-quantitative immunohistochemical assay using anti-HER2 antibody is applied to determine HER2 protein overexpression in breast cancer tissues. The specificity of the HER2 antibody (e.g. Clone4B5) is critical because the test results will help oncologist decide a patient should receive Herceptin® treatment. By using the 10K high-density protein microarray, we have revealed that the antibody 4B5 is not specific to HER2 protein. As shown in the graph above, this antibody also reacts with ZSCAN1B and HER4 (ERBB4). In contrast, HER2 UltraMAB (Clone UMAB36) only recognizes HER2 protein and is thus specific"
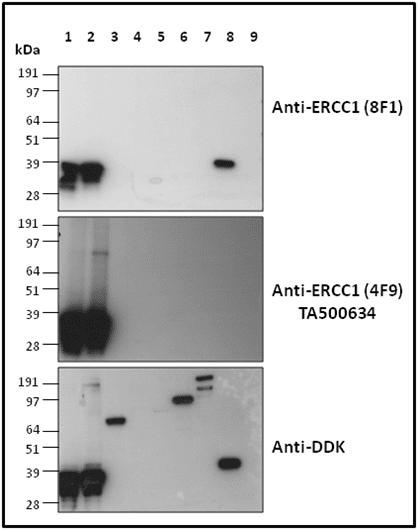
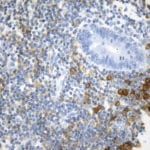
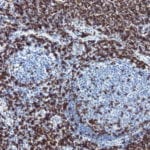
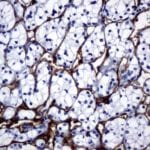
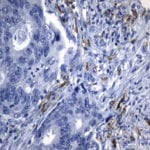
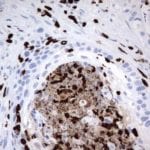
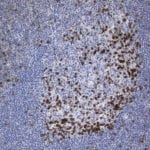
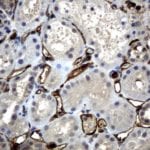
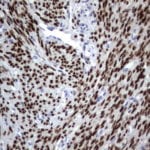
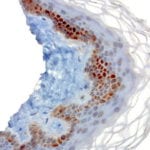
Case Study 2: ERCC1 IHC Antibodies
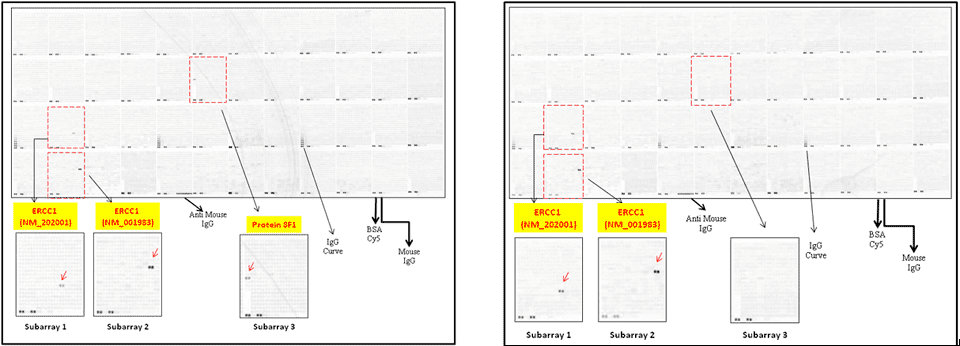
"The excision repair cross-complementation group 1 (ERCC1) protein is an important biomarker for clinicians to predict whether certain patient populations with non-small cell lung carcinoma will respond to cisplatin chemotherapy. As such, it is critical to develop highly-specific immunohistochemistry validated monoclonal antibodies for this diagnostic test. Several publications revealed that 8F1, the most commonly used antibody clone for ERCC1, exhibits cross-reactivity to an unknown protein in ERCC1 deficient cell lines. By using the high-density protein microarray technology, the corresponding cross-reactive binding protein for 8F1 monoclonal antibody was identified. This technology also enable us to successfully develop the most specific UltraMAB™ monoclonal antibody for ERCC1 (4F9, Clone UMAB8). This data was further confirmed by western blot analysis. Review the publication on the validation of ERCC1 antibodies.”