In the realm of genetic engineering, few breakthroughs have captured the imagination of scientists and the public alike as profoundly as CRISPR-Cas9. At the end of 2023, the first CRISPR-based gene therapy was approved by both the MHRA and the FDA. To commemorate this, our AMSBIO CRISPR expert, Dr Ifeanyi Enekwa, has written a blog to share some information, insights and thoughts for the future.

From Basics to Brilliance: AMSBIO’s Lab Glow-Up with BioPORTER®, Detachin™, and SoluLyse™
February 13, 2024
Win one of two $1000 Travel Grants for AACR 2024 or ISSCR 2024!
January 3, 2024What is CRISPR-Cas9 Gene editing?
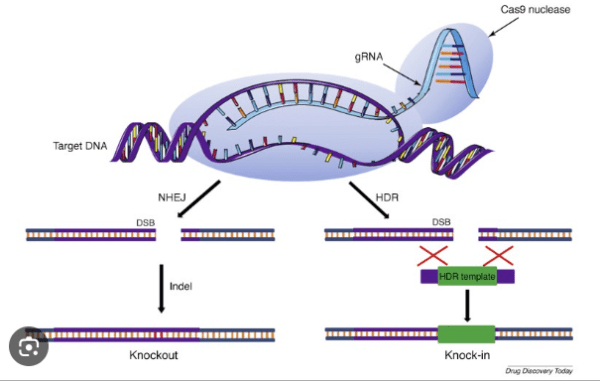
CRISPR-Cas9, an integral component of bacterial adaptive immunity, has emerged as a revolutionary tool in genetic engineering. Functioning as molecular scissors, its RNA and protein components collaborate to precisely cut mutated DNA strands, facilitating the seamless incorporation of normal DNA into targeted locations by scientists
MHRA and FDA Approvals for Sickle Cell Anemia
In a groundbreaking development, Casgevy, a collaborative effort between Vertex Pharmaceuticals (VRTX.O) and CRISPR Therapeutics (CRSP.BN), alongside bluebird bio’s (BLUE.O) Lyfgenia, recently received approval for the treatment of sickle cell anemia in individuals aged 12 and older.
The UK’s Medicines and Healthcare products Regulatory Agency (MHRA) granted approval on November 16, 2023, marking the world’s first CRISPR-based gene therapy. Subsequently, on December 8, 2023, the U.S. Food and Drug Administration (FDA) endorsed two gene therapies for sickle cell disease, marking a historic milestone as the first applications of the Nobel Prize-winning CRISPR gene editing technology in the United States. Notably, these gene therapies, Casgevy and Lyfgenia, represent the inaugural FDA-approved drugs utilizing CRISPR-Cas9, ushering in a new era of cell-based treatments for sickle cell disease.
These therapies involve the modification of patients’ hematopoietic stem cells through ex-vivo genome editing using CRISPR/Cas9 technology, followed by the reinfusion of these modified cells into the patient. The significance of this achievement extends beyond sickle cell disease, holding promise for addressing monogenic diseases like certain genetic variations of Cystic fibrosis and Beta Thalassemia caused by nucleotide deletions and point mutations.
Looking into the future for gene-editing therapies
However, while this milestone is a giant leap forward, the path to widespread clinical implementation faces hurdles. According to a report by GlobalData, only 24 CRISPR-based drugs are currently in Phase 2 trials, with the majority still in earlier-stage or preclinical development. Safety concerns loom large, as off-target cutting by CRISPR-Cas9 could lead to genetic lesions and unintended diseases. Yet, recent breakthroughs in the CRISPR gene-editing platform, such as base-editing and prime-editing, show promise in mitigating these safety concerns by minimizing DNA double-strand breaks and unwanted mutations.
The recent approval’s visibility is expected to positively influence funding for institutions engaged in gene-editing research, potentially expediting the technology’s development. While the future of gene-editing appears bright, caution is warranted. Addressing existing challenges is paramount before this transformative technology can firmly establish itself in clinical medicine, paving the way for a new era of targeted and personalized genetic therapies.
AMSBIO and CRISPR
Clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9 is the latest technology revolutionizing the field of genome editing. CRISPR/Cas9 allows for sequence-specific gene editing in a flexible and simple system resulting in high specificity and low cell toxicity.
AMSBIO offers a comprehensive selection of products to support your genome editing research including CRISPR kits, vectors and gene knockout kits. In addition we offer a custom service to design and clone guide RNA and donor vectors tailor-made to suit the needs of your project.
For more information or support, contact us on [email protected]. We’d love to hear from you!