Categories
Biotin labelled
A selection of biotinylated proteins with superb bioactivity and high detection sensitivity
Growth factors & cytokines
An extensive range of recombinant cell signaling molecules for cell culture
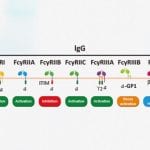
Immunotherapy Proteins
Purified, soluble immunoreceptors involved in key immunosignaling pathways
Epigenetics
A range of nucleosomes, histones, bromodomains, DNA & histone modifying enzymes as well as small molecule epigenetic modulators
Kinases
Purified human recombinant protein & lipid kinases for consistent, reliable results