Our Services
Our synthetic mRNA service provides you with Ψ-containing mRNA which is translated more efficiently than its unmodified counterpart and results in higher levels of protein expression in certain translational systems. The combination of enhanced levels of protein expression and avoidance of the innate immune response against exogenous RNAs makes in vitro synthesized mRNA an attractive option for the induction of protein synthesis in desired cells.
Simply provide us with the desired sequence data and we will synthesize your high quality synthetic mRNA by in vitro transcription.
Our custom synthetic mRNA service includes the following:
-
-
-
-
- Synthesis of synthetic DNA
- Plasmid cloning
- Purification & characterization
- RNA synthesis by in vitro transcription
- Further purification & characterization
-
-
-
Our synthetic mRNA is made to research grade for development applications, and GMP grade for cell therapy.
Production Steps
- Chemical synthesis of dsDNA fragments
- Cloning of the DNA fragments in a plasmid
- Quality control by sequencing of plasmid inserts
- Synthesis of PCR primers, based on the plasmid sequence, for PCR amplification of the cloned insert sequence. If desired, a 3’-Poly(A) tail can be included.
- PCR amplification of the cloned insert with the primers described in step 4
- Purification of the PCR products with spin columns
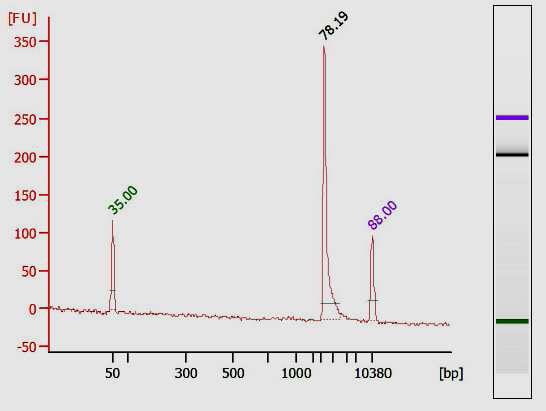
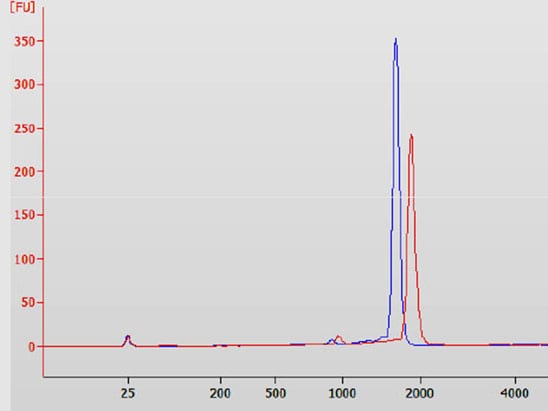
- Quality control of PCR products by capillary electrophoresis (Agilent Bioanalyzer)
- PCR templates for production of ssRNA by in vitro transcription with T7 RNA polymerase
- DNase treatment for removal of the DNA template
- Purification of ssRNA with spin columns
- Quality control of the in vitro transcripts by capillary electrophoresis (Agilent Bioanalyzer)
Molecular Biology Request Form

Let's get started
Complete our service request form and we will contact you to discuss your requirements.