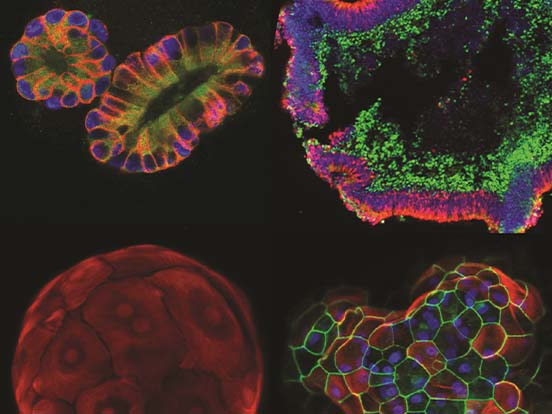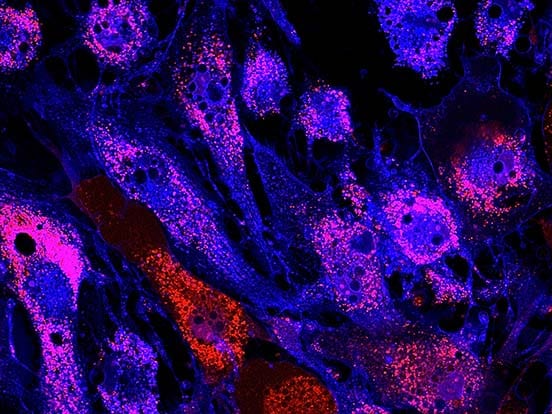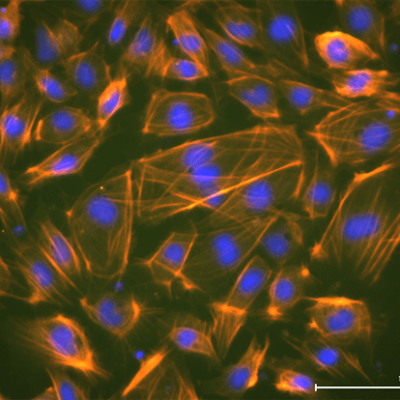
Image courtesy of Dr. Toyohara (Tohoku University, Sendai, Japan)
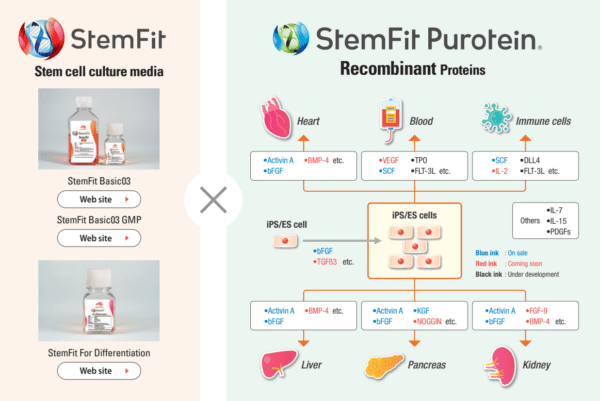
StemFit Purotein® Recombinant Proteins
Animal-origin free, GMP-grade recombinant cytokines and growth factors
StemFit Purotein® is a range of animal-origin free recombinant cytokines and growth factors that play key roles in the differentiation of ES and iPS cells into liver, pancreas, kidney, heart, blood and immune cells. Available in GMP and non-GMP formats and PMDA approved for cell therapy products, StemFit Purotein® recombinant proteins enable a seamless transition from basic to clinical research.
AMSBIO’s StemFit Purotein® and media ranges, when used together with iMatrix laminin and CELLBANKER series freezing solutions, create the perfect Stem Cell Synergy for your ES/iPS cell culture.
Benefits
- Animal-origin free
- GMP compliant Activin-A and bFGF available
- High Purity & Performance
- Reliable lot-to-lot consistency
- Ready-to-use frozen formulation, simplifying protocols
- Regulatory compliance for cell and gene therapy product manufacturing*
*Approved by Japanese PMDA- equivalent to FDA
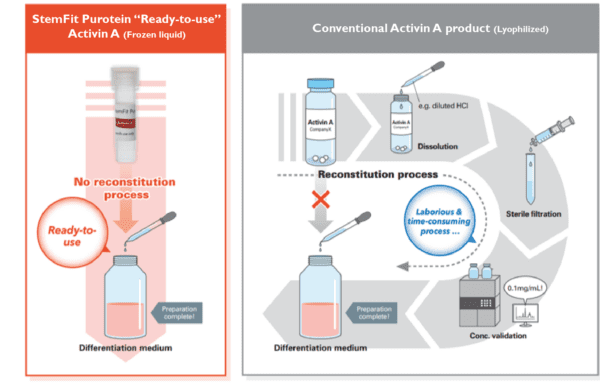
Ready-to-use Frozen Format
Conventional lyophilized products require reconstitution and concentration measurements which are time-consuming and introduce the risk of bacterial contamination or unexpected inactivation. StemFit Purotein® recombinant proteins are provided in a ready-to-use frozen liquid formulation, eliminating reconstitution steps. This feature simplifies the cell manufacturing process and accelerates cell therapy projects.
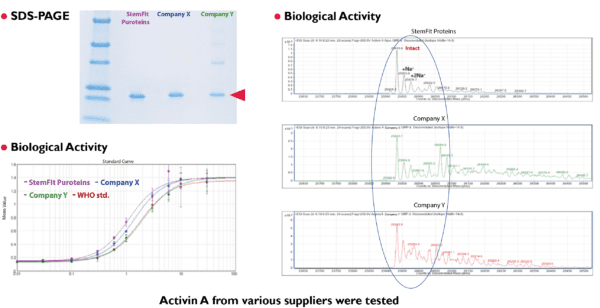
High lot-to-lot consistency
StemFit Purotein® recombinant proteins have been manufactured using Corynebacterium glutamicum expression systems. Since C. glutamicum secretes expressed target proteins into culture media, highly purified proteins can be obtained with a simple purification process, eliminating the need for costly and time-consuming refolding. For proteins expressed in other expression systems requiring refolding, we have implemented a cutting-edge refolding technology, FMR (Flow Microreactor). FMR enables high purity and high-performance protein production, minimizing undesirable effects from impurities and ensuring consistent lot-to-lot results.
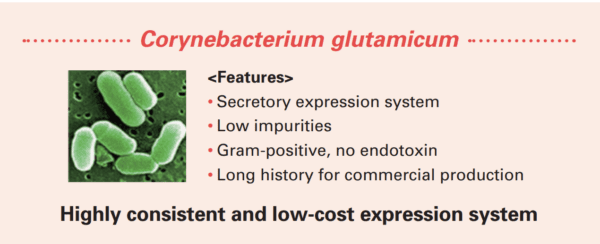
Highly consistent and cost-effective Corynebacterium glutamicum expression system
Corynebacterium glutamicum is a gram-positive, non-sporulating soil bacterium that has successfully been used in the industrial production of amino acids for over 50 years. Since C. glutamicum secretes expressed target proteins into culture media, highly purified proteins can be obtained with a simple purification process. Additionally, gram-positive bacteria contribute low endotoxin products.
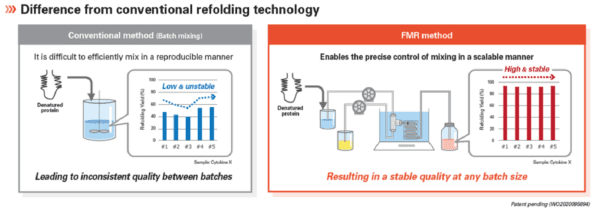
Cutting Edge Flow Microreactor (FMR) Refolding Technology (for Activin A)
Refolding is an important process in the production of recombinant proteins since it has a significant impact on product quality. Typically, a batch mixing process is used where denatured proteins and refolding buffer are mixed in a flask in a strictly controlled manner. Using this conventional method, it is difficult to efficiently mix sample and buffer in a reproducible manner, leading to inconsistent quality between batches.
StemFit Purotein® recombinant Activin A is expressed in E.coli and manufactured with an advanced refolding technology, FMR (Flow Microreactor), in which protein and buffer are continuously flowed and mixed in micro space. FMR allows for the precise control and optimisation of the mixing reaction at a micro-second scale, enabling efficient and consistent refolding between lots and different manufacturing scales.
Animal-Origin Free and Regulatory Compliance for CGT
Animal and human-derived components are known to carry a risk of hazardous viral contamination for cell therapy. Therefore, StemFit Purotein is designed and manufactured under a strict animal-origin free policy and is free from animal and human-derived components, according to the requirements of the Japanese PMDA (equivalent to the FDA) for ancillary materials. Additionally, StemFit Purotein® offers GMP compliant products that are manufactured under GMP guidelines and are thus preferable for cell therapy product manufacturing.
High compatibility with StemFit hPSC culture media and iMatrix ECM
Human pluripotent stem cells (hPSCs) are promising resources for research and cell therapy since they can differentiate into various types of cells. In the directed differentiation process of hPSCs, cells undergo several differentiation steps towards the target tissues. During this process, recombinant proteins are added to the culture media as growth factors to stimulate differentiation. StemFit hPSC media and iMatrix recombinant ECM are suitable for all stages of research and highly compatible with StemFit Purotein®. The combination of StemFit hPSC media with StemFit Diff Differentiation Supplement and StemFit Purotein® allows for the establishment of highly efficient differentiation systems in the laboratory while ensuring an easy transition to GMP-compliant production for future cell therapy manufacturing
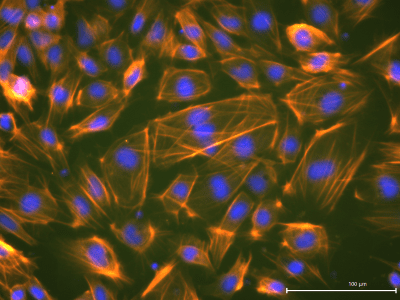
StemFit Puroteins® in Action
Dr. Toyohara and colleagues at Tohoku University in Japan have successfuly used our StemFit Purotein Activin A in their differentiation cocktail to generate vascular smooth muscle cells (vSMCs) from iPSCs!
vSMCs form a strong and functional layer around our blood vessels, with vSMC dysfunction leading to an increased risk of morbidity. These iPSC-derived vascular smooth muscle cells have many exciting applications in regenerative medicine, from disease modelling to tissue engineering, to improve our understanding of their role in disease and how to treat patients.
Product Information Table
| Name | Datasheet | Packsize | Order |
|---|---|---|---|
| StemFit Purotein Human recombinant Activin A, GMP | 1 mg | View | |
| StemFit Purotein Human recombinant Activin A, non-GMP, 10ug/tube | 10 ug (0.1 mg/ml, 100 ul) | View | |
| StemFit Purotein Human recombinant Activin A, non-GMP, 1mg/tube | 1 mg | View | |
| StemFit Purotein Human recombinant Activin A, non-GMP, 50ug/tube | 50 ug (0.1 mg/ml, 500 ul) | View | |
| StemFit Purotein Human recombinant FGF Basic, GMP Compliant | 1 mg (0.3 mg/ml) | View | |
| StemFit Purotein Recombinant Human BMP-4, Non GMP | - | 10 ug (0.1 mg/ml, 100 ul) | View |
| StemFit Purotein Recombinant Human KGF (FGF-7), Non GMP | 1 mg (0.1 mg/ml, 10 ml) | View | |
| StemFit Purotein Recombinant Human KGF (FGF-7), Non GMP | 10 ug (0.1 mg/ml, 100 ul) | View | |
| StemFit Purotein Recombinant Human KGF (FGF-7), Non GMP | 50 ug (0.1 mg/ml, 500 ul) | View | |
| StemFit Purotein Recombinant Human SCF (Non GMP) | 1 mg (0.1 mg/ml, 10 ml) | View | |
| StemFit Purotein Recombinant Human SCF (Non GMP) | 10 ug (0.1 mg/ml, 100 ul) | View | |
| StemFit Purotein Recombinant Human SCF (Non GMP) | 50 ug (0.1 mg/ml, 500 ul) | View | |
| StemFit Purotein Recombinant Human VEGF 165 (Non GMP) | 1.0 mg (0.1 mg/ml, 500 ul) | View | |
| StemFit Purotein Recombinant Human VEGF 165 (Non GMP) | 10 ug (0.1 mg/ml, 100 ul) | View | |
| StemFit Purotein Recombinant Human VEGF 165 (Non GMP) | 50 ug (0.1 mg/ml, 500 ul) | View |