Features
- Transfect both dividing and non-dividing cells
- No host-genome integration & stable expression
- Ease to produce at high viral titre (helper free)
- Does not elicit significant immune response in vivo
Applications
- Measuring transduction efficiency
- For use as a negative control
- Transduction for in vitro applications
- Injection for in vivo applications
What is an Adeno-Associated Virus?
Adeno-associated virus (AAV) is a non-enveloped, single-stranded DNA virus which is approximately 20nm in size and can infect both dividing and non-dividing cells.
AAV does not cause disease and elicits a very mild immune response. Being able to infect both dividing and non-dividing cells, it incorporates its genome into that of the host cells and only replicates in the presence of a helper virus; most commonly adenovirus or herpes simplex virus.
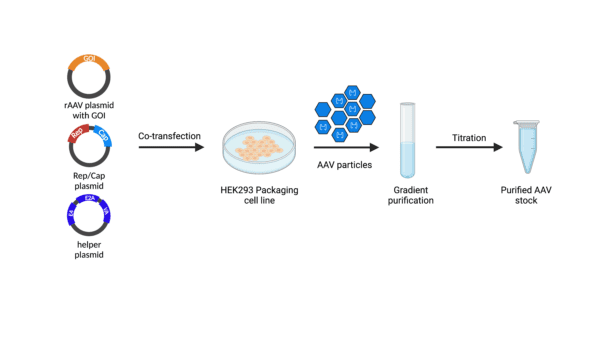
Our Products
Our collection of over 2,000 AAV controls is made up of various genetic elements, packaged into different serotypes in multiple combinations. The table below shows the options we have available.
Available options
| Promoters | ALB, aMHC, c-Fos, CAG, CaMKIIa, CK0.4, CK1.3, CMV, cTnT, EF1a, EFFS, GFAP, HCRApoE, MBP, MCK, MeCP2, NSE, PDX1, PGK, Rpe65, SST, Syn, TBG, UBC |
| Reporters | GFP, mCherry, RFP, LacZ, Luciferase |
| Inducible reporters | DIO-GFP, DIO-mCherry, DIO-RFP, DIO-LacZ |
| Inducible sysyems | Cre, DIO, tTA |
| CRISPR/Cas9 | Cas9, gRNA scaffold |
| Serotypes | AAV1, AAV2, AAV5, AAV6, AAV7, AAV8, AAV9, AAV-DJ |
See all AAV Particles
Unsure which serotype to use?
If you are unsure which serotype will work best with your application, try our AAV GFP Testing Kit. This kit contains CMV-GFP in AAV1, AAV2, AAV5. AAV6. AAV8, AAV9, and AAV-DJ serotypes so you can test the efficiency on different cell types.
| Name | Datasheet | Packsize | Order |
|---|---|---|---|
| AAV GFP Testing Kit/ different serotypes | 7 x 50 ul | View |
Frequently asked questions
The adeno-associated virus (AAV) is a small, icosahedral and non-enveloped virus that belongs to the Parvoviridae family. A helper virus, such as adenovirus or Herpes virus is usually required for a productive infection to occur. AAV does not encode its own polymerase so its replication process
relies on host cell polymerase activities.
Recombinant AAV is artificial AAV without any AAV rep or cap genes which encode viral replication and structural proteins, respectively. In rAAV, rep and cap are replaced with a gene or construct of interest flanked by the ITRs for replication and packaging. Efficient packaging of rAAV can be performed with constructs ranging from 4.1kb to 4.9kb in size.
As AAV is a replication-deficient virus, its replication depends on other helper viruses such as adenovirus and herpes virus.
AAV has the capacity to produce high titre virus in dividing and non-dividing cells and the potential for long-term gene transfer with minimum immunogenecity.
rAAV constructs that encode no tumorigenic gene and are produced in the absence of a helper virus can be handled in a Biosafety Level 1 (BSL-1) facility. Otherwise, it should be handled as biohazardous material under Biosafety Level 2 (BSL-2) containment.
To date, AAV is not linked to any human disease. For wild type AAV, replication is at an extremely low efficiency without the presence of a helper virus, like adenovirus. The rAAV is composed of several plasmids (cis plasmid, Helper plasmid, rep/cap plasmid). The cis plasmid and helper plasmid do not share any regions of homology with the rep/cap gene-containing plasmid; this means the likelihood of a rAAV being able to replicate is theoretically impossible.
AAV has a packaging capacity of around 4.7Kb. When the length of the DNA inserted between the two ITRs is close to the maximal allowed, 4.7Kb, the packaging efficiency decreases significantly. For instance, for gene over-expression from cDNA, since the CMV-poly(A) signal element is about 1Kb,
the maximum allowable cDNA length is about 3.2Kb. Whereas if GFP co-expression (about 0.8Kb) is considered, the allowable capacity is about 2.4Kb.
We currently provide serotypes AAV1, AAV2, AAV5, AAV6, AAV7, AAV8, AAV9, and AAV-DJ. Please see the guide below to determine which would be best for your application.
| Tissue Type | Recommended AAV Serotypes |
|---|---|
| Muscle | AAV1, AAV6, AAV8, AAV9 |
| Liver | AAV8, AAV9, AAV-DJ |
| Lung | AAV5, AAV6, AAV9 |
| Central Nervous System | AAV1, AAV5, AAV8, AAV9, AAV-DJ |
| Retina | AAV1, AAV2, AAV5, AAV8 |
| Pancreas | AAV8 |
| Kidney | AAV2, AAV9 |
| Heart | AAV1, AAV8, AAV9 |