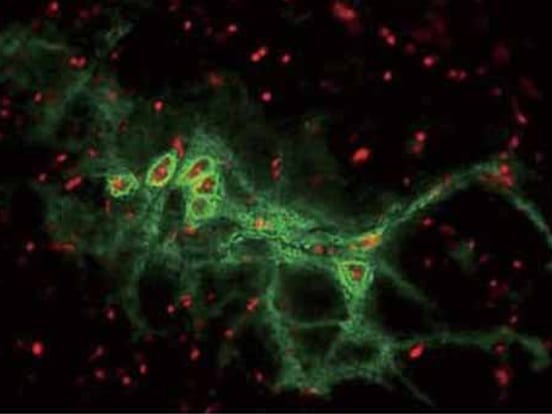
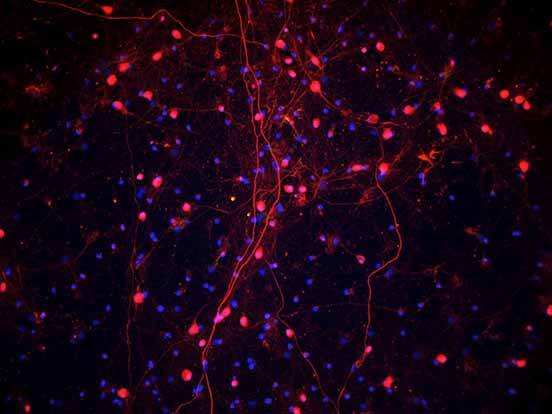
Scientists at Keio University Hospital have successfully transplanted iPS cell-derived neural stem/progenitor cells (iPSC-NS/PCs) into a patient, to start the first-in-human clinical study for the treatment of spinal cord injury using iPSC-derived cells.

MiRNA as a Novel Therapy for Myocardial Fibrosis
March 21, 2022
US Research reveals the role of Heparan Sulfate in Obesity
March 1, 2022Research Breakthrough
The team led by Hideyuki Okano, professor of physiology at Keio University, received approval by the Japanese government for the project in 2019, but patient recruitment had to be suspended due to the covid-19 outbreak. The group has now successfully transplanted human iPSC-NS/PCs into the first participant of the study. (The target sample size for this initial study is four patients.)
The first purpose of the study is to evaluate the safety of cell transplantation in patients with subacute spinal cord injury. The effectiveness is also being evaluated side by side.
The transplanted cells were prepared at a Good Manufacturing Practice (GMP) grade cell processing facility at Osaka National Hospital using a clinical-grade integration-free hiPSC line established by the Kyoto University Center for iPS Cell Research and Application (CiRA). Cells are cryopreserved at Keio University Hospital using STEM-CELLBANKER
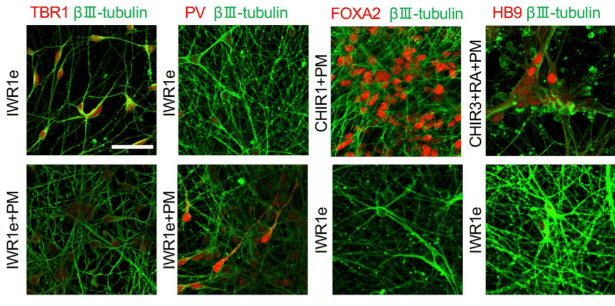
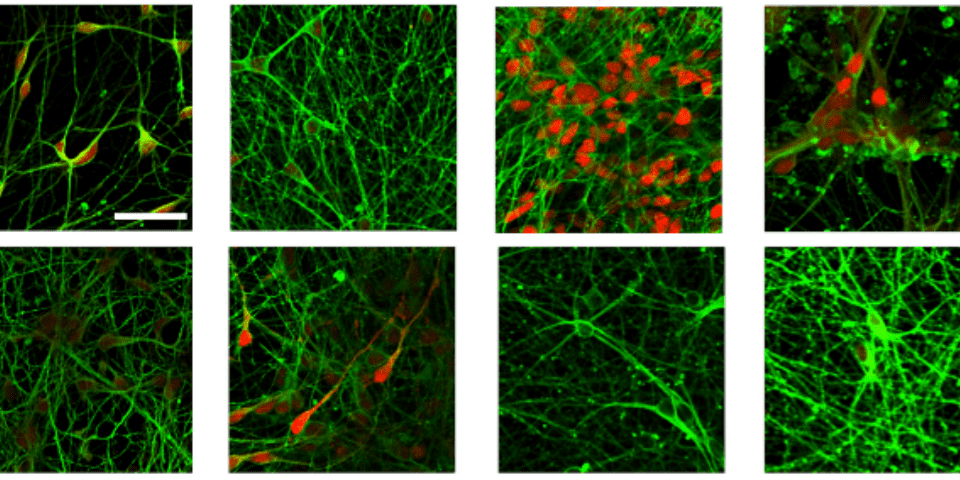
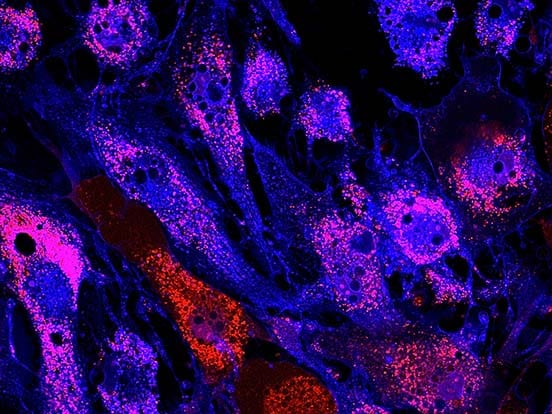
Ps: Image in fig 1 is reproduced from Sato, T. et al. (2021). Neuroscience Letters, 746, 135676. Generation of region-specific and high-purity neurons from human feeder-free iPSCs. under a Creative Commons license (CC BY 4.0).
CiRA’s protocol features stem cell culture in StemFit cell culture medium with iMatrix-511 MG recombinant Extracellular Matrix together with cryopreservation in STEM CELLBANKER. All three reagents are available globally through AMSBIO, creating the perfect Stem Cell Synergy for ES/iPS cell culture in research use or GMP settings
The surgery is the first transplant of human iPS cell-derived neuronal progenitor cells for spinal cord injury. Based on the data up to about 3 months after transplantation to the first patient, an independent Monitoring Committee of experts will assess the treatment’s safety. The team will proceed with the second transplant, if the committee confirms it is safe to continue the project. As a result, they are currently suspending the recruitment of the following participants, and the resumption of recruitment will be expected to be around March.
At a press conference, Hideyuki Okano, the research group leader, said “I am glad to come to this stage after overcoming various difficulties.”
Future Research Highlights
- The university is now monitoring the patient, who is currently undergoing physical rehabilitation at Murayama Medical Center in Tokyo.
- The university plans are to recruit the next patient in April at the earliest. As for prospects for the treatment’s practical use, the university said it is likely to take “at least three to five years” to obtain the data needed for it.
- About 2 million cells are transplanted into each patient in the treatment. They were created from iPS cells stored at Kyoto University in western Japan, according to Keio University.
- In the future, the university plans to increase the number of cells to be transplanted in order to enhance the effectiveness of the treatment.
- Some 5,000 people sustain spinal cord injuries every year in Japan and the number of people living with spinal cord injuries is said to exceed 150,000.
- The research team has also unveiled a plan to start research within the next few years using iPS cells to confirm the safety and efficacy in treating patients in the chronic stage.
Citations
First-in-human clinical trial of transplantation of iPSC-derived NS/PCs in subacute complete spinal cord injury: Study protocol. Sugai, K. et al. (2021). Regenerative Therapy, 18, 321-333.
Generation of region-specific and high-purity neurons from human feeder-free iPSCs. Sato, T. et al. (2021). Neuroscience Letters, 746, 135676.
https://www.keio.ac.jp/en/press-releases/files/2022/1/14/220114-1.pdf
Stem Cell Synergy from AMSBIO – Streamlined and efficient ES/iPS Cell Culture for basic to clinical research. https://www.amsbio.com/stem-cell-synergy/
CiRA Protocol for Human iPS cell culture under feeder-free conditions – https://www.cira.kyoto-u.ac.jp/e/research/img/protocol/Ff-iPSC-culture_protocol_E_v140311.pdf
Need Help Finding a Product?
Our reagent specialists are to help you find the best product for your application. Please Call or Email us and we will be happy to help you find the right product for the job.
Subscribe to our newsletter
Be the first to hear about upcoming events, product news, special offers, application notes, and more