Obesity is an increasingly common metabolic disease that affects over 40% of adults and 18% of children and adolescents in the United States. It is an independent risk factor for the development of many other diseases, including type 2 diabetes, cardiovascular disease, and cancer. In the context of obesity, various cytokines activate macrophages in white adipose tissue (WAT), promoting chronic inflammation that disrupts glucose, lipid, and energy metabolism. Despite these significant breakthroughs, scientists still don’t fully grasp how macrophages and adipocytes cooperate to maintain metabolic balance.
World’s First Spinal Cord Injury Treatment Using iPS cells
March 7, 2022
AMSBIO Prize-Winning Poster – UK Proteoglycans 2022
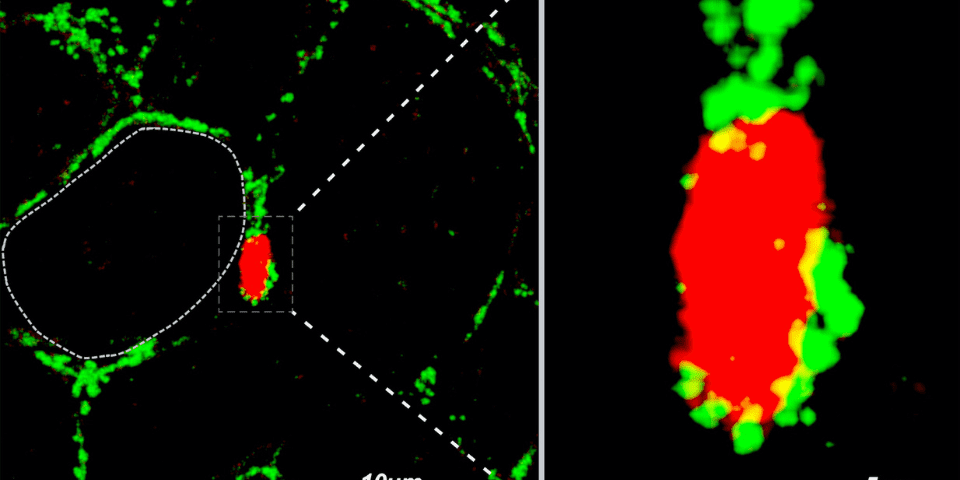
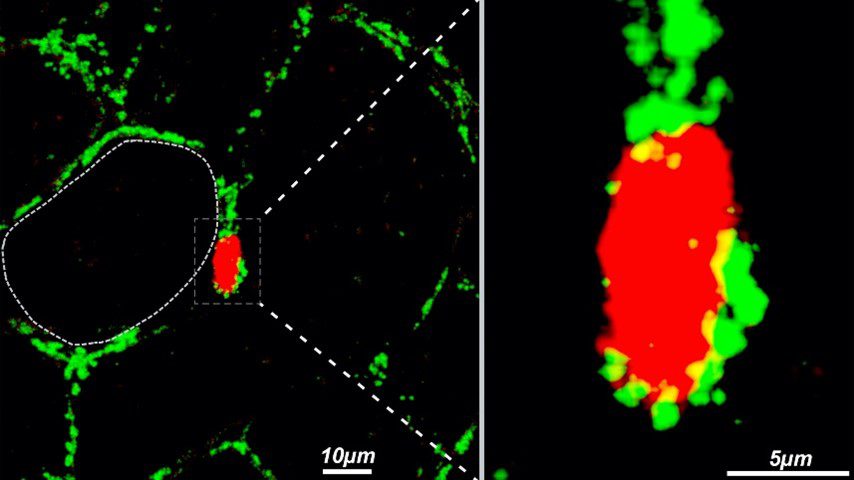
February 17, 2022Recent research suggests that mitochondria can be transferred between cells to help metabolically compromised cells survive. However, it is still unknown if intercellular mitochondria transfer happens in WAT or whether it controls metabolic homeostasis in vivo. Researchers from the Brestoff and Teitelbaum Labs at Washington University School of Medicine, St. Louis (USA) demonstrated that macrophages acquire mitochondria from nearby adipocytes in vivo, defining a transcriptionally different macrophage subpopulation. A genome-wide CRISPR-Cas9 knockout screen showed that heparan sulphates are required for mitochondrial uptake. Obese mice due to a high-fat diet (HFD) have lower HS levels on WAT macrophages and reduced intercellular mitochondria transfer from adipocytes to macrophages. According to their findings, macrophages in White Adipose Tissue (WAT) can internalize mitochondria from other cell types in vivo (See fig.2).
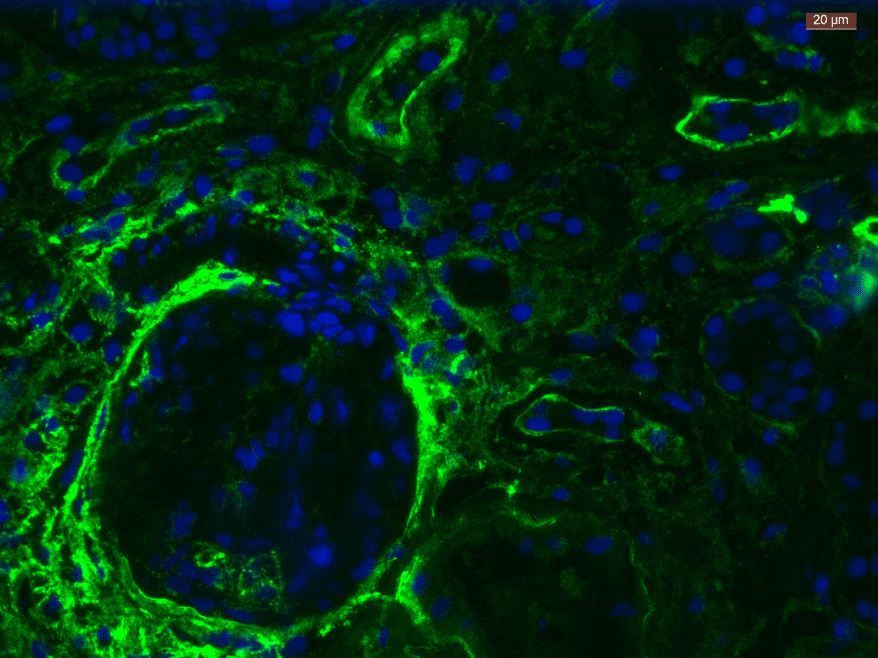
For heparan sulfate quantification on cells, AMSBIO supplied the scientific team with the anti-HS antibody (clone: F58-10E4). The flow cytometry data with the 10E4 illustrated that mitochondria uptake depends on heparan sulfates.

Jonathan Brestoff, Assistant Professor, at the Department of Pathology and Immunology, Washington University School of Medicine, St. Louis (USA), has concluded that:
Mitochondria are the power plants of cells, and it has long been assumed that they are made in one cell and never leave. We discovered that isn’t really the case and found that fat cells give some of their mitochondria to an immune cell type called macrophages. In obesity, this transferring of mitochondria between cells goes awry, contributing to faster weight gain and worse metabolism. Using a tool called CRISPR, we screened the entire genome and figured out that cells trade mitochondria using a special type of sugar called heparan sulfates, which we think acts like a loading dock for receiving cargo like mitochondria. When we delete heparan sulfates on macrophages, mice get fat. This suggests to us that it’s probably good for cells to trade mitochondria with each other. My lab is now trying to figure out how this mysterious and surprising process of mitochondria transfer works because we believe we can harness this biology to treat some human diseases.
Key Points
- Dr Wentong Jia, a postdoctoral fellow at the Brestoff lab added “The cell surface expression of heparan sulfate, a glycosaminoglycan required for mitochondria uptake in macrophages, depends on a key glycosyltransferase named EXT1. The 10E4 antibody from AMSBIO has helped us verify that we’ve successfully prevented Heparan Sulfate from being synthesized in cells that lack EXT1.”
- “I find it fascinating that cells use heparan sulfates to take up mitochondria,” says Rocky Giwa, a PhD candidate in the Brestoff Lab. “I wonder if there’s a correlation between the amount or composition of heparan sulfates and a cell’s ability to efficiently take up mitochondria from other cells. Since the various HS antibodies have unique specificities, the different clones can help us start to attack that question.”
Further reading
- For further information on heparan sulfate antibody (clones: 10E4, 3G10, and JM403) please visit https://www.amsbio.com/heparan-sulfate-antibodies/ or contact AMSBIO on+44-1235-828200 / +1-617-945-5033 / [email protected].
- Published paper (PumX metric went up to top 1% for citations for all Cell Metabolism papers that published in the last 1-year).
- Brestoff, J. R., Wilen, C. B., Moley, J. R., Li, Y., Zou, W., Malvin, N. P., … & Teitelbaum, S. L. (2021). Intercellular mitochondria transfer to macrophages regulates white adipose tissue homeostasis and is impaired in obesity. Cell metabolism, 33(2), 270-282. Available online at ScienceDirect: https://www.sciencedirect.com/science/article/pii/S1550413120306033
- Borcherding, N., Jia, W., Giwa, R., Field, R. L., Moley, J. R., Kopecky, B. J., Chan, M. M., Yang, B. Q., Sabio, J. M., Walker, E. C., Osorio, O., Bredemeyer, A. L., Pietka, T., Alexander-Brett, J., Morley, S. C., Artyomov, M. N., Abumrad, N. A., Schilling, J., Lavine, K., … Brestoff, J. R. (2022). Dietary lipids inhibit mitochondria transfer to macrophages to divert adipocyte-derived mitochondria into the blood. Cell Metabolism, 34(10), 1499-1513.e8. https://doi.org/10.1016/j.cmet.2022.08.010. Available online at Science Direct: https://www.sciencedirect.com/science/article/pii/S1550413122003539