In an innovative study in 2020, researchers Young Sun Hwang, Shinnosuke Suzuki, Yasunari Seita, and colleagues from the Sasaki lab devised a protocol in which induced pluripotent stem cells (iPSCs) could be utilised to produce reconstituted human testes. This exciting development allowed the Sasaki lab to recapitulate human male germ cell development in vitro, exposing the cellular mechanisms that occur in the foetal and early postnatal stages of life.
Cutting edge technology for vascularized kidney organoids
February 17, 2023
Investigating Glycans – shedding new light on the ‘dark matter’ of biology
January 10, 2023Non-obstructive azoospermia (NOA) is characterised by the failure of spermatogenesis and affects 10-15% of infertile men. Hwang and colleagues’ research brings us closer to understanding the processes that result in NOA by modelling prospermatogonial specification from iPSCs. Their novel study reliably and accurately produced human prospermatagonia in vitro and observed the expression of transposable elements that indicate development. The strides made by researchers in the Sasaki lab have paved a new way for future studies to explore treatments for male infertility.
StemFit (available from AMSBIO) was used in this study to maintain iPSC culture. StemFit is a range of feeder-free, weekend-free and chemically defined stem cell culture media suitable for basic and clinical research. The cells were cultured on plates coated in recombinant laminin E8 fragments using iMatrix-511 silk (available from AMSBIO). iMatrix-511 has become the gold standard for the maintenance and expansion of stem cell culture, as demonstrated by Hwang et al. in their protocol. The Sasaki lab also cryopreserved their cells using CELLBANKER 1 from AMSBIO, a trusted solution for the storage of any cell type including sensitive cell lines. CELLOTION™ cell wash and recovery solution from AMSBIO was also used by the lab to collect FACS-sorted in vitro cells prior to single-cell RNA-seq library preparation.
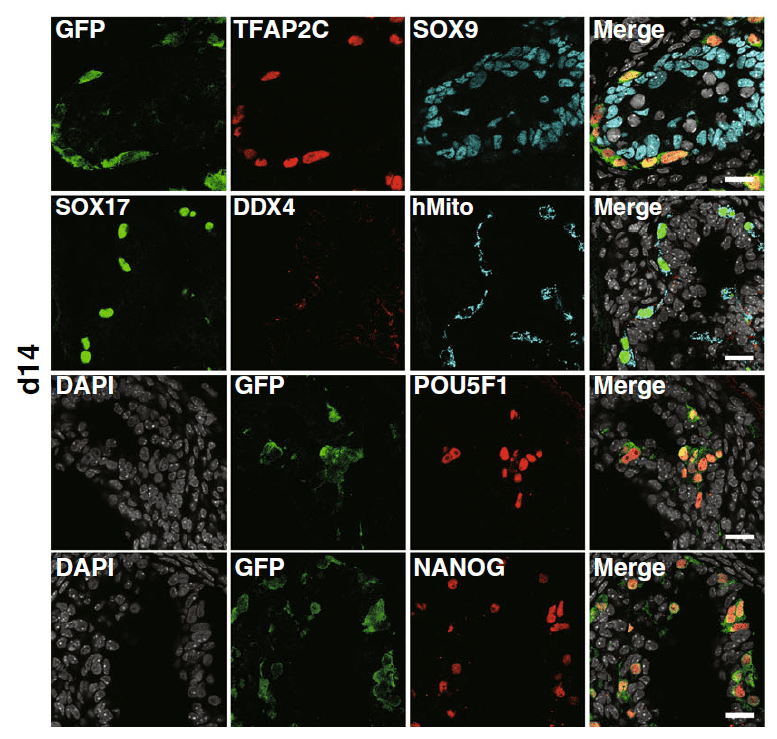
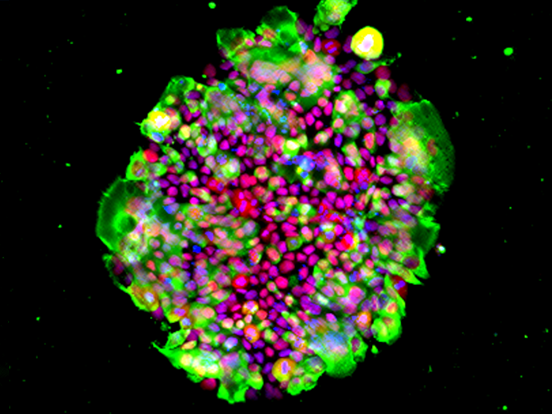
Figure 1. Immunofluorescent images of d14 xenogeneic reconstituted testes for germ cell markers (red: TFAP2C, DDX4, SOX17, POU5F1 or NANOG), markers for human PGCLC-derived cells (green: GFP; cyan: hMito), a Sertoli cell marker (cyan:SOX9), and DAPI (white), with their merges. Scale bars, 20 µm. (Licensed under a Creative Commons
Attribution 4.0 International License)
The gene regulatory networks affecting germ cell development amongst mammals are highly diverse and integral to the health and prosperity of a species. In 2022, researchers from the Sasaki lab used the same iPSC line and stem cell culture protocol established in the previous study again, utilising StemFit and iMatrix-511 silk, to maintain and grow their iPSC cells. This study by Jumpei Ito and colleagues characterised the transcriptomes of naïve pluripotent stem cells and primordial germ cell -like cells (PGCLCs), which highlighted a common gene regulatory network between the two cell types. These findings indicate a group of hominid-specific endogenous retroviruses, LTR5_Hs, are responsible for similarities in gene expression between iPSCs and PGCLCs. Ito and colleagues suggest that the insertion of LTR5_Hs was key during evolution, setting in motion the genetic changes that separated primates from other mammals.
AMSBIO is the global supplier of StemFit, iMatrix-511 and CELLBANKER. Our Stem Cell Synergy Solution offers a streamlined and efficient way to propagate, maintain, and store your stem cells, for basic research through to clinical applications. For quick and simple long-term storage of your cells, we recommend STEM-CELLBANKER cryopreservation medium to ensure high cell-viability post-thaw. Available in GMP-grade formulations, STEM-CELLBANKER retains cell identity and allows for direct freezing at -80°C. Find out more about how Stem Cell Synergy Solution could improve your research here.
References
- Reconstitution of prospermatogonial specification in vitro from human induced pluripotent stem cells
Hwang, Y.S. et al. Nature communications, 2020, 11(1), 5656.
Ito, J. et al. PLoS Genet, 2022, 18(5): e1009846.
Stem Cell Synergy Citations
Sano, E. et al. iScience, 2021, 24(5), 102428-102428
Miyazaki, T. et al. Scientific reports, 2017, 7, 41165
Cui, D. et al. Frontiers in cell and developmental biology, 2021, 9, 656867-656867