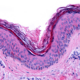
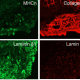
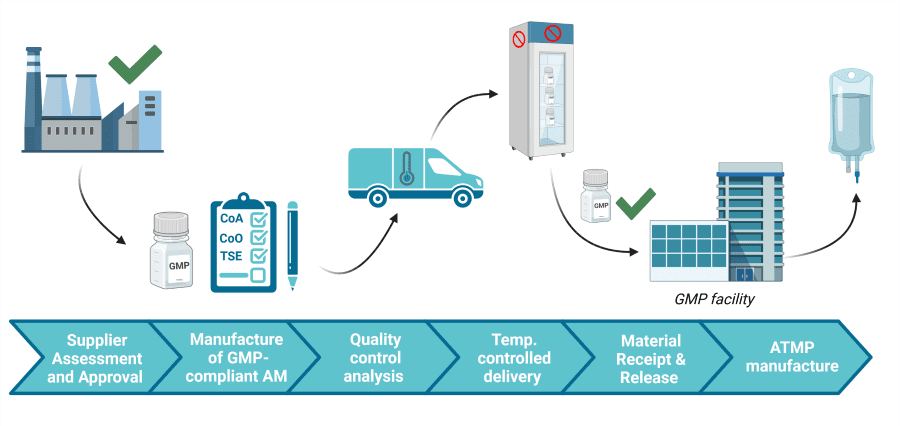
Advanced Therapy Medicinal Products (ATMPs), such as cell and gene therapy products, are envisioned to be the therapies of the future. Good Manufacturing Practice (GMP) establishes the highest possible manufacturing standards for these innovative medical products.
GMP guidelines are followed to ensure the highest quality & safety of both the cells (starting materials), and reagents you add to grow, change and store these cells (ancillary materials). However, the world of GMP-compliant materials and when to switch from research-grade reagents is complex!
We talked to AMSBIO customer & GMP expert Dr. Erik Miljan at BIODIVIDE Ltd. to explain & unravel GMP for our customers, to ensure a streamlined clinical translation.