Our Services
Integrase Deficient Lentivirus Construction
-
-
-
-
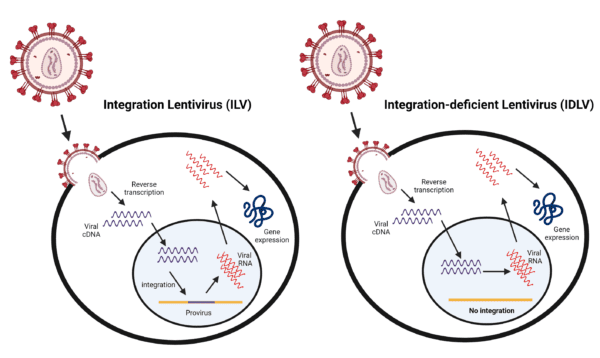
- Non-integrative lentiviral vector derived from HIV-1 with a class 1 integrase (IN) mutation (replacement of the 262RRK motif by AAH)
- Either you provide target templates or we synthesize or obtain it from a cDNA collection
- You can choose the promoter, a tag, a selection marker and a fluorescent marker
- We construct the integrase-deficient lentiviral transfer plasmids and generate ready-to-use IDLV's with your target gene inserted
-
-
-
Features:
-
-
-
-
- Reduce the risk of insertional mutagenesis
- Transient gene expression in dividing cells and stable gene expression in non-dividing cells
- Custom-tailored lentiviral transfer plasmid to fit each specific project
- Gene therapy studies
-
-
-
Why use AMSBIO's lentiviral services?
-
-
-
-
- Safe-to-use (self-inactivating) lentiviral particles can deliver your gene into a wide range of cell lines including non-dividing, primary or stem cells
- Engineered in-house lentiviral vectors allow for highly efficient gene integration into the cell genome
- You can choose to have an inducible or constitutive expression
- High titer lentivirus that is monitored by a fluorescent protein (not fused with your target)
- Our experts have years of experience in lentiviral cloning and expression
- Fast turnaround time
- The best price and the best quality in its class
-
-
-
Safety Precaution
Please note that although our lentiviral vectors contain all necessary bio-safety features, work with lentiviral particles should be carried out under Biological Safety Level 2 (BL2) or higher. Please conduct a thorough risk assessment for your project and contact your health and safety facilities for local guidelines and regulations.
Lentivirus & Adenovirus & AAV Request Form

Let's get started
Complete our service request form and we will contact you to discuss your requirements.