AMSBIO's Guide to GMP
The importance of GMP in Regenerative Medicine.
Good Manufacturing Practice (GMP) is a system for ensuring that products are consistently manufactured to a high quality and safety standard.
GMP is a central topic of discussion in the regenerative medicine community, as following GMP guidelines is crucial for the manufacture of an innovative new class of pharmaceuticals: Advanced Therapy Medicinal Products e.g. cell and gene therapy products.
AMSBIO is an ISO 9001 registered firm and we understand the importance of GMP compliance for Regenerative Medicine & Cell Therapy. Furthermore, we recognize the complexity researchers face in the switch from research-grade to GMP-compliant materials in your ATMP development.
Here at AMSBIO, we can provide expertise and the highest quality ancillary materials to streamline your clinical translation.
What is GMP?
GMP guidelines are in place to ensure consistency, high quality, and traceability of a final product e.g. a pharmaceutical product or medical device.
What does it actually mean to be GMP compliant?
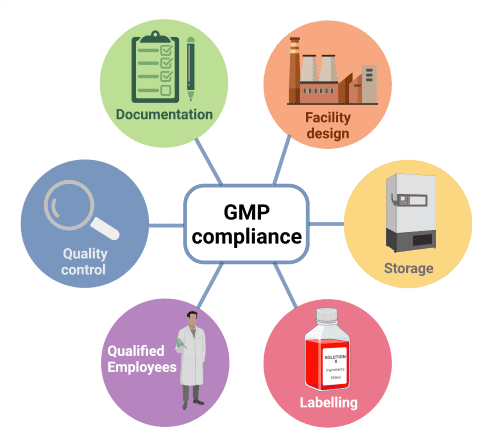
GMP encompasses all processes involved in the manufacture of a product, including quality control/assurance, documentation, employee training and the design of the manufacturing facility (Fig. 1).
The final outcome is a reliable, high-quality product with low lot-to-lot variability, which has been stored and labelled correctly.
GMP & ATMP Development
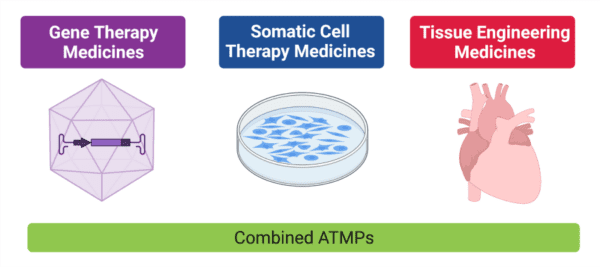
What are ATMPs?
Advanced Therapy Medicinal Products (ATMPs) are defined as medical products for human use that are based on genes, tissues, or cells e.g. cell and gene therapy products. These innovative medical products are expected to become the therapies of the future for currently untreatable diseases and injuries.
Acceleration in the ATMP sector
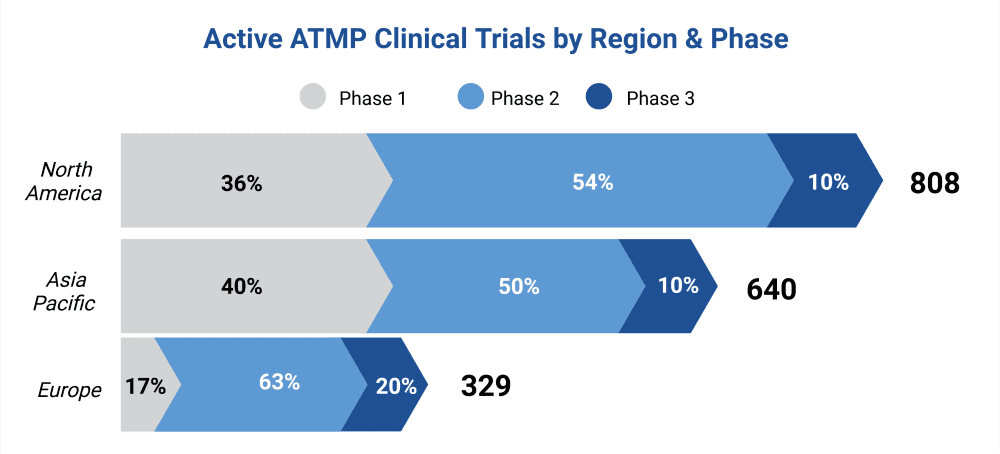
In North America, Europe, and Asia there are currently around a total of 1,777 active clinical trials investigating ATMPs, with significant commericial investment into the ATMP sector (Fig.3). (Source: Alliance for Regenerative Medicine).
Why is GMP important in ATMP development?
As ATMPs are intended for human use, ATMP manufacture falls under pharmaceutical law, meaning GMP guidelines must be followed.
Additionally, due to the biological nature of cell and gene therapy products, ATMP developers face particularly unique regulatory challenges.
To overcome these regulatory hurdles, it is crucial that ATMP manufacturers follow strict GMP guidelines, to produce a high quality, functional and safe medical product.
Advent Bioservices are an AMSBIO customer and a cell therapy contract development & manufacturing organisation (CDMO), with state-of-the-art GMP facilities for the manufacture of ATMPs.
We asked what GMP meant to their company:
Here at Advent Bioservices, GMP covers the hygiene and sanitation of out premises, the validation of our equipment, the quality of the reagents and consumables we use and the training and capability of our personnel carrying out activities within the facility. Our priority is the patient we are manufacturing the medicine for. We want the medicine to be safe, consistent, to do exactly what it should and documented for traceability from start to finish.
GMP-compliant Ancillary Materials
In contrast to conventional pharmaceuticals, every starting material (e.g. the cells) and every reagent utilised to produce an ATMP is, in effect, part of the final ATMP.
These reagents are called ancillary materials (AMs) and they include reagents such as cell culture media, substrates, and enzymes. AMs pose an inherent risk to the safety, potency, and purity of the final ATMP, and therefore the quality and consistency of AMs is paramount.
Click here to read our new blog post with Dr. Erik Miljan all about the importance of being GMP Ready with a secure supply of AMs in your ATMP research!

At what stage in ATMP development should researchers transition to GMP-compliant AMs?
It is now advised to use GMP-grade AMs, instead of research-grade AMs, early in the R&D/pre-clinical phases of ATMP development.
This is because any changes in ancillary materials after crucial evidence has been collected will mean the completion of costly comparative experiments to ensure consistency. This step will cost extra time & money, in an already stressful period of progressing your new therapy into clinical trials.
What regulations apply to ancillary materials?
There are currently no AM-specific regulations in worldwide regulatory frameworks. However, there are many regulatory organisations (see box) that provide guidance on strategies to control AMs, and many AM suppliers comply with GMP guidelines to streamline the ATMP manufacturing process.
Regulatory organizations
- FDA = Food and Drug Administration (US)
- EMA = European Medicines Agency (EU)
- MHRA = Medicines & Healthcare products Regulatory Agency (UK)
- PMDA = Pharmaceuticals and Medical Devices Agency (Japan)
- ICH = The International Council for Harmonisation
- ISO = International Organization for Standardization
- PIC/S = Pharmaceutical Inspection Co-operation Scheme
What documentation should accompany GMP-compliant AMs?
Approved AM suppliers provide evidence in the form of documentation to demonstrate that the product has met defined quality and performance standards. Documentation is reviewed when the AM arrives at a GMP facility, and the AM is only released into the facility if the specifications are to standard.
Robust documentation to record all elements of raw materials sourcing and manufacturing is key in demonstrating quality and safety to regulators.
Important Documents
- Certificate of Analysis (CoA) = Does the product meet defined quality and performance standards?
- Certificate of Origin (CoO) = Where do the materials used to make the AM come from?
- TSE-Certificate = Is the AM supplier minimising the risk of TSE-contamination in animal-derived products?
- Qualification documents = Is the supplier's equipment and facility fit for purpose?
- Drug Master File (DMF) = Do the FDA have detailed, confidential information about the supplier's AM on file, ready to review with an application?
- Stability documents = Has the AM shipping/storage conditions been validated by ICH studies?
How does a DMF work?
-
The AM supplier will draft a Letter of Authorisation (LoA) and a copy is sent to the GMP customer & the FDA. This allows the FDA to access the Drug Master File for review as part of the overall regulatory review of the customer's application.
The Life of a GMP-compliant Ancillary Material!
Check out the figure below to learn the steps taken to ensure a secure GMP supply chain!
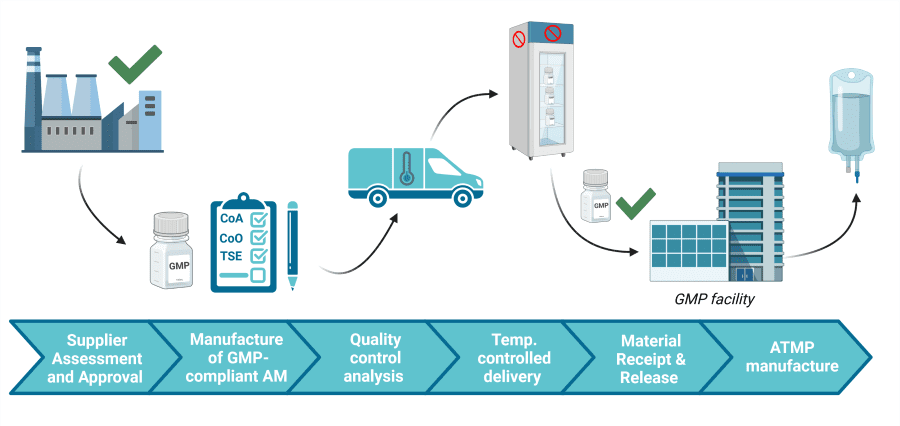
AMSBIO can help you be GMP ready!
- AMSBIO can facilitate a seamless pre-clinical to clinical transition, reducing the risk of regulatory delays!
- Establish a secure supply of high quality & reliable, GMP-compliant materials for your ATMP manufacturing.
- Support for your change control procedures & quality risk management.
- FDA-registered Drug Master Files available, for streamlined clinical translation.
- AMSBIO is ISO 9001 certified, allowing you to have confidence in your GMP supply chain.
- Ensured traceability: all required documentation available upon request.
Discover our range of high quality, GMP-compliant Products & Services today!
Disclaimer: AMSBIO's GMP-compliant Products & Services are not pharmaceuticals/therapeutic products, and are only intended for manufacture of investigational ATMPs. They are not suitable for direct administration to humans.
References
Simplifying and Standardizing Organoid Cryopreservation.
Thought Leadership Piece in Today’s Clinical Lab (Jun 29, 2022: 3 min read). Written by Zahraa Chorghay, PhD.
Current and Future innovations in stem cell technologies.
Miljan, E., Hadlington-Booth, W. (2022, June 10). Labmate Online.
Advanced therapy medicinal products: Overview
European Medicines Agency, (2022).
Cell and Gene Therapy Catapult ATMP clinical trials report 2021.
Catapult: Cell and Gene Therapy, (2022).
Regenerative Medicine: The Pipeline Momentum Builds.
Alliance for Regenerative Medicine, (2022).
Advanced Therapy Medicinal Products: What's in a Name?
Jiskoot, W. (2020), Journal of Pharmaceutical Sciences, 109(11), 3282, 3284.
Considering Cell Therapy Product "Good Manufacturing Practice" Status.
Bedford, P., Jy, J., Collins, L., & Keizer, S. (2018). Frontiers in medicine, 5, 118-118.
Regenerative Medicine and Regulation: what's GMP got to do with it?
EuroStemCell, (2016).
Manufacturing process development of ATMPs within a regulatory framework for EU clinical trial & marketing authorisation applications
Detela, G., Lodge, A., (2016). Cell & Gene Therapy Insights, 2(4), 452-452.
Current perspectives on the use of ancillary materials for the manufacture of cellular therapies.
Solomon, J., Csontos, L., Clarke, D., Bonyhadi, M., Zylberberg, C., McNiece, I., . . . Deans, R. (2015). Cytotherapy (Oxford, England), 18(1), 1-12.
Figures created using Biorender.com